Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 9
23 October 2019
Frederic Hoerr, DVM, MS, PhD
Veterinary Diagnostic Pathology, LLC
638 South Fort
Valley Road
Fort Valley, Virginia, 22652 USA
CASE I: WSC18-19 #1 (JPC 4117005).
Signalment: Three-year-old Peahen (Pavo cristatus)
History: A reportedly three-year-old peahen was presented deceased for necropsy examination following a weeklong history of being lethargic with no mobility in her legs. She was eating and drinking well. The peahen had whitish diarrhea few days before death. The other 11 birds in the group were acting normally.
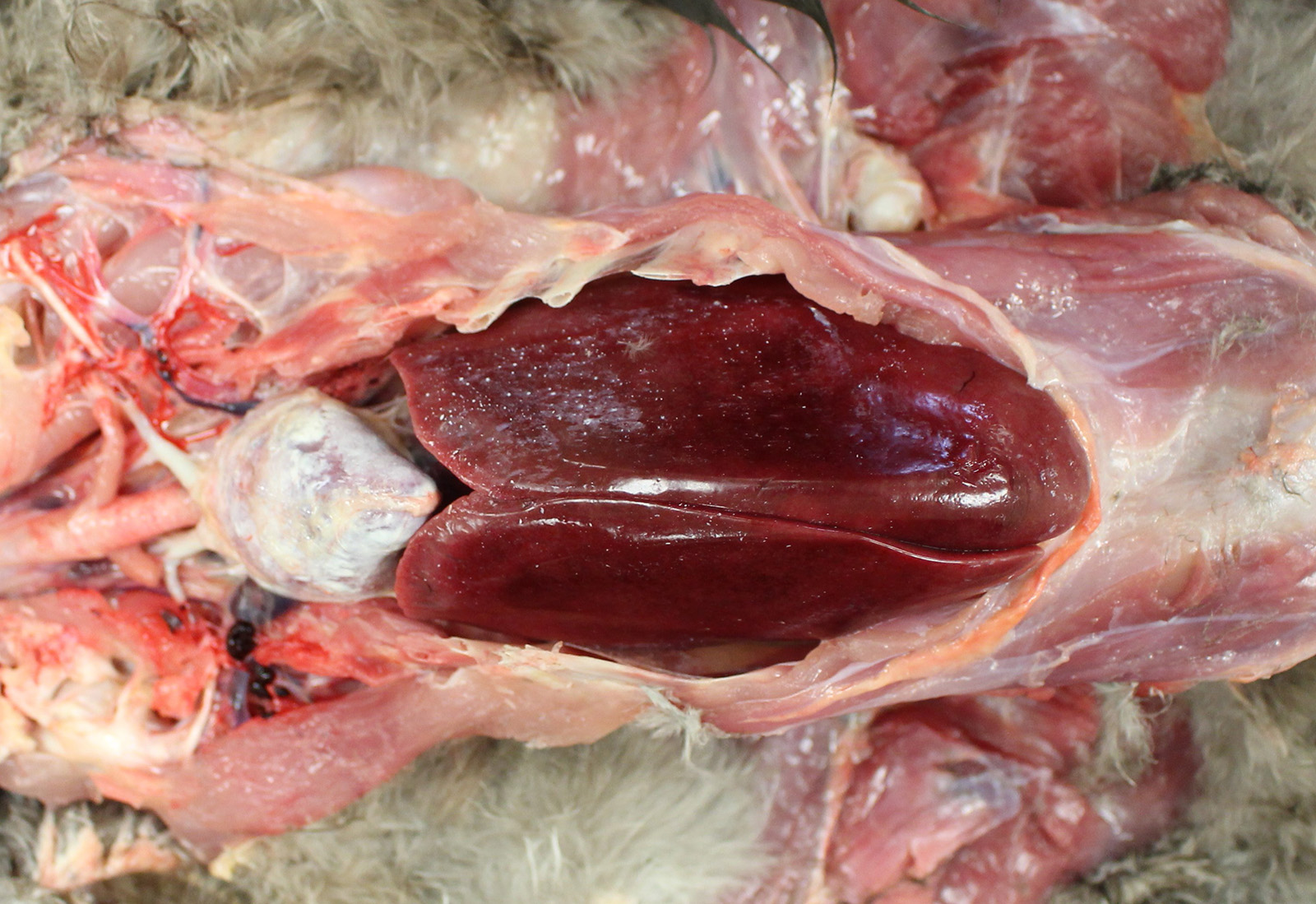
Gross Pathology: The bird was in good body condition with adequate pectoral muscling. Diffusely, the pericardial sac was mildly thickened by a large amount of white chalk-like material which was adhered to its surface. The kidneys appeared pale and on cut section, there was white granular material throughout the renal parenchyma.
The inner wall of the coelomic cavity and the surfaces of the visceral organs including the liver and abdominal air sacs were covered in small quantities of fine white powder-like material. The duodenal contents were mixed with white granular material throughout.
The right stifle joint had significant subcapsular hemorrhage that extended into the adjacent muscle. Subcutaneously and intramuscularly along the medial aspect of the left tibiotarsus were extensive dark red areas (hemorrhage).
No significant gross lesions were seen in the oral cavity, trachea, lungs, esophagus, crop, proventriculus, gizzard, jejunum, caecum, colon, uterus, ovaries, liver, lymph nodes, spleen or brain.
Laboratory results: The pooled liver and kidney sample was positive for Marek´s disease virus by real-time PCR.
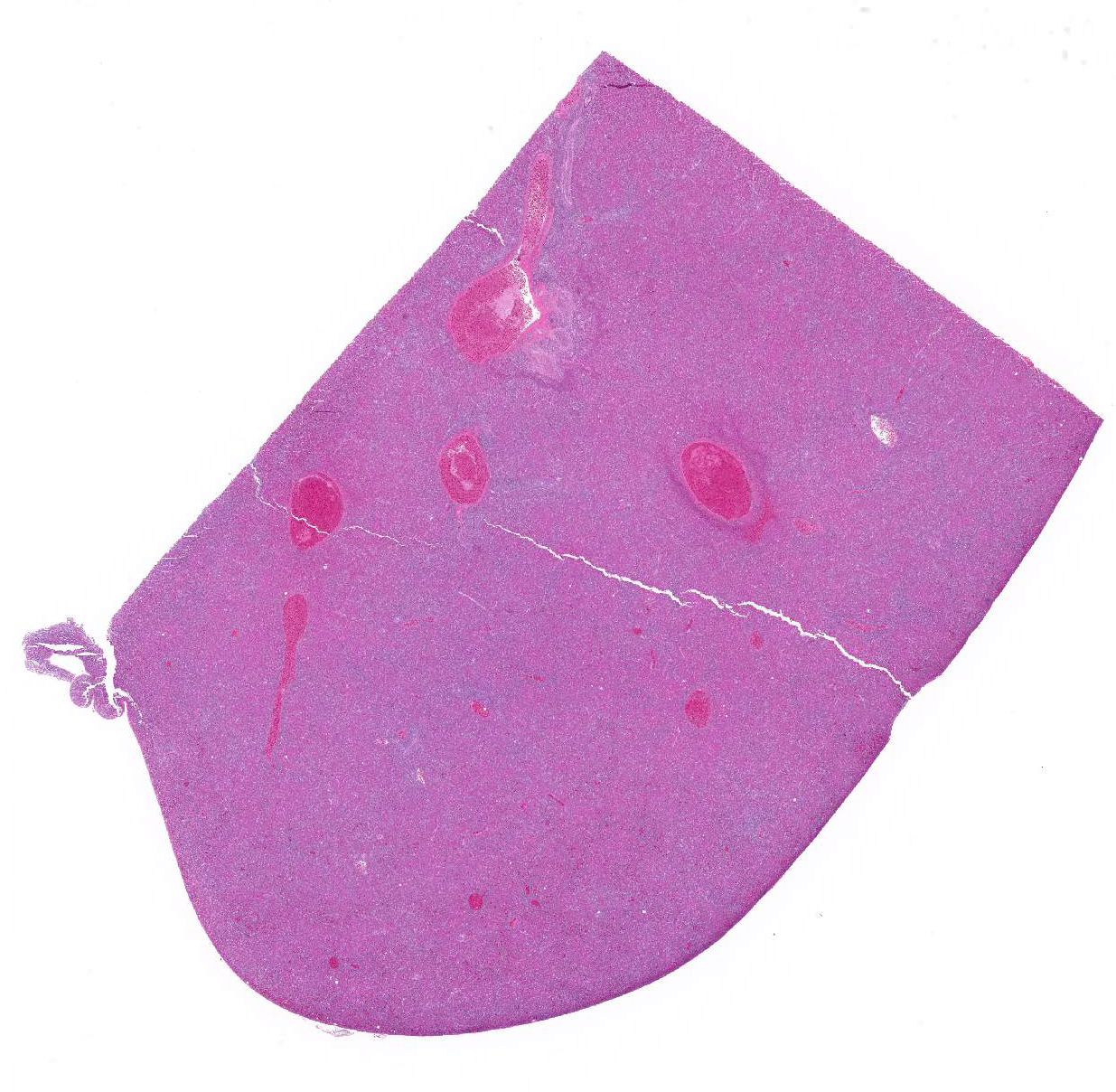
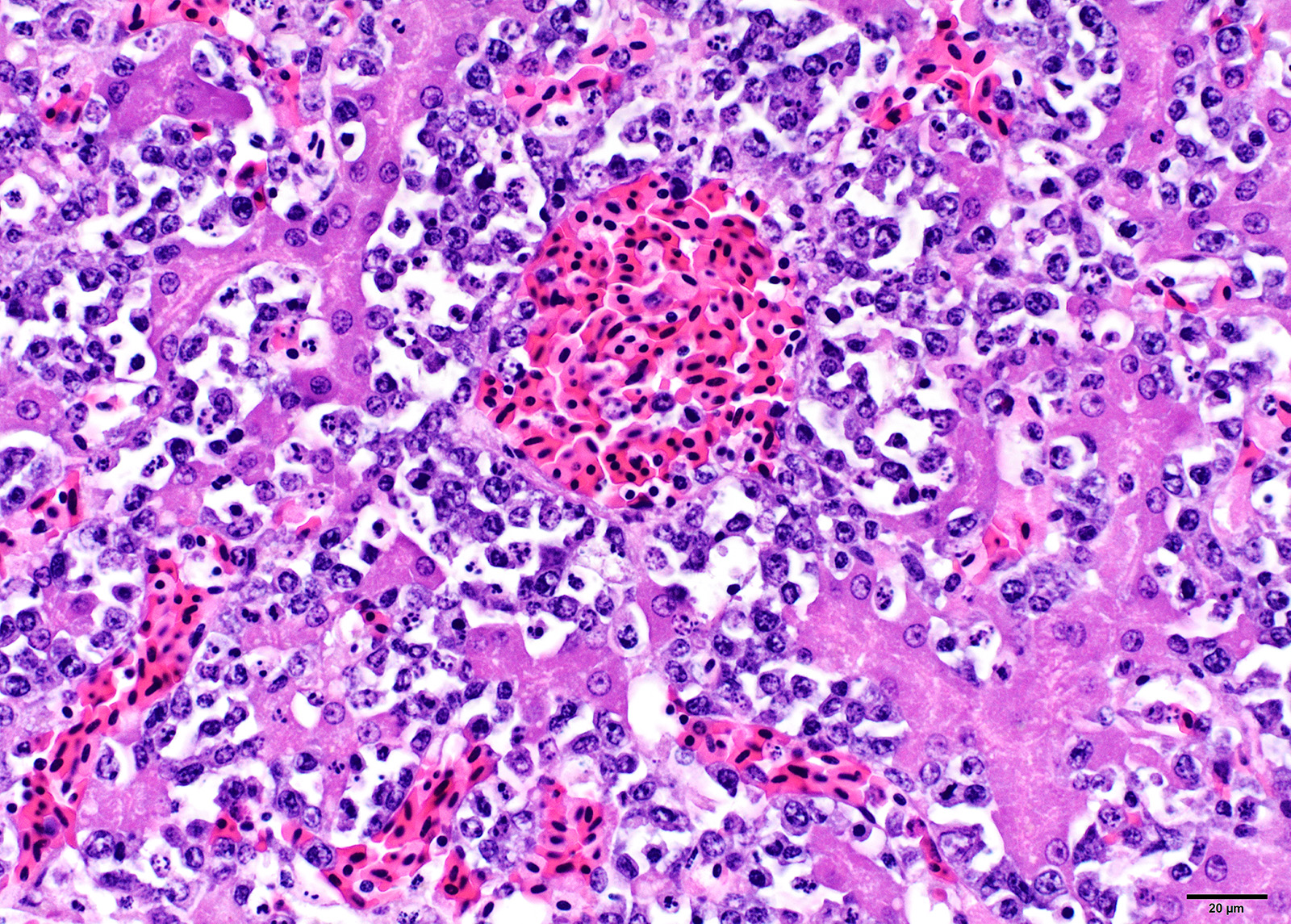
Microscopic Description: Liver: Diffusely, the normal hepatocellular architecture is disrupted by an accumulation of large numbers of medium to large sized lymphocytes and lymphoblasts that moderately expand the sinusoidal spaces. The pleomorphic lymphoid cells are more concentrated around the central veins (centrilobular area). These lymphoid cells contain small to moderate amounts of basophilic to amphophilic, homogenous cytoplasm with round to oval nuclei with coarsely stippled chromatin and indistinct nucleoli. Approximately 20-25% of the neoplastic lymphocytes appear degenerate with fragmented nuclei that appear as basophilic pyknotic bodies of variable sizes. There are approximately 3-5 mitotic figures per HPF (400X). Multifocally, the hepatocytes are mildly compressed by the infiltrating lymphoid cells and occasionally few hepatocytes show single cell necrosis with hypereosinophilic cytoplasm, pyknotic nuclei, and a clear space around them.
Similar lymphoid infiltrates were present in the kidneys, proventriculus, ventriculus, small intestine, ceca, spleen, ovary, and globes.
Immunohistochemistry:
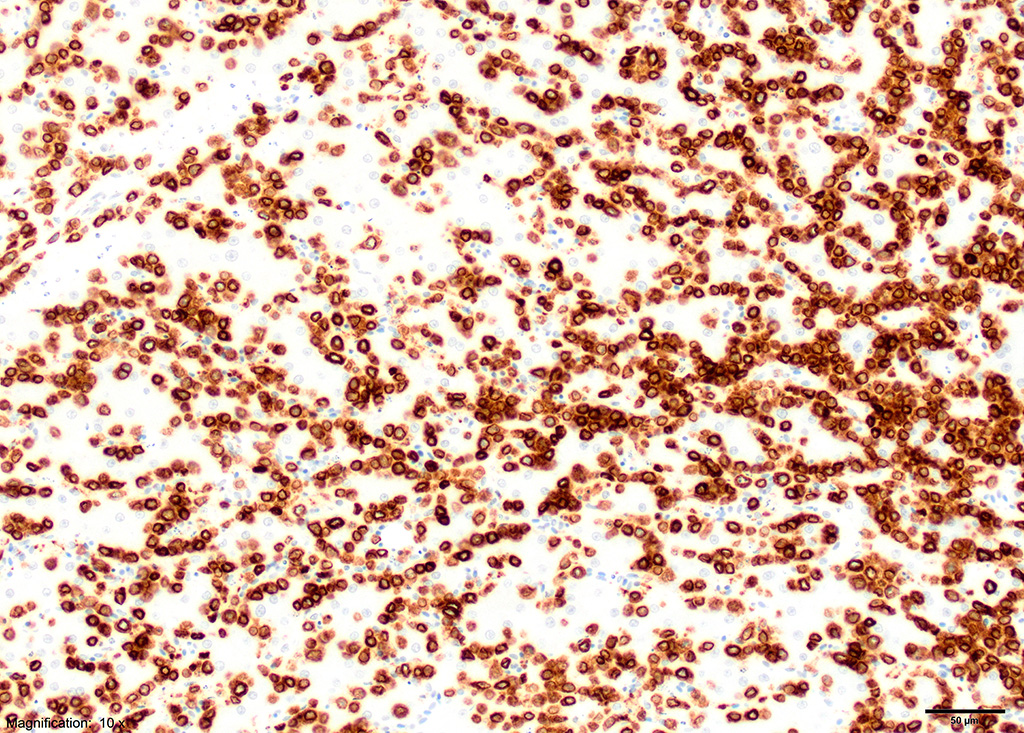
Immunohistochemical staining showed strong positive reactivity of the lymphocytes for CD3 and negative for CD20 and CD79a.
Contributor Morphologic Diagnosis:
Liver: Lymphoid infiltration, diffuse, severe, with scattered hepatocyte necrosis.
Contributor Comment: Liver: Diffusely, the normal hepatocellular architecture is disrupted by an accumulation of large numbers of .
The gross lesions of visceral gout with deposition of urates on the heart, liver, and kidneys were most likely the result of impaired renal function caused by infiltration of neoplastic lymphocytes. Many neoplastic diseases in poultry are caused by viral etiologies. A list of the viral induced neoplastic diseases seen in poultry are provided in Table 1.
|
Table 1: Transmissible neoplasms 2. |
|||
|
Virus type |
Nucleic acid type |
Virus classification of etiological agent |
Neoplastic diseases |
|
Retrovirus |
RNA |
Leukosis/sarcoma Group
|
Leukoses Lymphoid leucosis Erythroblastosis Myeoloblastosis Sarcomas and other connective tissue tumors Fibrosarcoma, fibroma Myxosarcoma, myxoma Osteogenic sarcoma, osteoma Histiocytic sarcoma Related neoplasms Hemangioma Nephroblastoma Hepatocarcinoma Osteopetrosis |
|
Reticuloendotheliosis Group (REV) |
Reticuloendotheliosis Lymphoid leucosis |
||
|
Herpesvirus |
DNA |
Marek´s disease virus |
Marek´s disease |
(Table adapted from Swayne DE, ed., Diseases of Poultry 2017.)
Confirmatory diagnosis of the visceral form of Marek´s disease requires histopathological examination, identification of MDV, by PCR and/or demonstration of MATSA antigen on tumor cells by immunohistochemistry.
Marek´s disease is a cell-associated lymphoproliferative disease that is commonly seen in domestic chickens and less commonly in other birds. This is believed to be the first report of Marek´s disease in this species of peafowl. A novel herpesvirus that was most closely related to gallid herpesvirus-3 was previously reported in three peafowls that had hepatocellular necrosis with intranuclear eosinophilic inclusions.9
The causative agent of Marek´s disease, gallid herpesvirus
2, belongs to the genus Mardivirus, subfamily: Alphaherpesvirinae and
family: Herpesviridae. The genus Mardivirus consists of different
serotypes: Serotype 1 MDV (gallid herpesvirus type 2), Serotype 2 MDV (gallid
herpesvirus type 3) and Serotype 3 herpesvirus of turkeys (meleagrid
herpesvirus type 1). Serotypes 2 and 3 are non-oncogenic.7 The
serotype-1 MDV consists of Marek´s disease virus of varying pathogenicity based
on which they are classified as mild (mMDV), virulent (vMDV), very virulent
(vvMDV) and very virulent + (vv+MDV).8 Clinical signs can occur in
chickens as early as 4 weeks of age and are commonly seen in birds between 12
and 24 weeks of age. The disease occurs in four forms:7
1) Classical form: Mainly characterized by neurological signs with partial or complete paralysis of legs and wings. Other common clinical signs include torticollis, dilation of crop, gasping, and respiratory distress depending on the nerves affected.
2) Acute form: Involves the formation of lymphomas in the visceral organs causing anorexia, depression, weight loss, and diarrhea.
3) Acute cytolytic form: Commonly seen in infections caused by vvMDV strains. It is characterized by high mortality with severe atrophy of lymphoid organs.
4) Transient paralysis: This is an uncommon form that lasts for about 24-48h and is usually associated with edema of the brain causing varying degrees of ataxia, paresis or paralysis of the legs, wings and neck.
Following infection by direct or indirect aerosol route
through inhalation of cell-free virus particles within feather dander, the
pathogenesis of MDV is very complex and is influenced by the age, immune
status, and genetic susceptibility. MD pathogenesis is characterized by four
phases:3,10
1) Early cytolytic phase: seen within 2-7 days post infection (dpi). Initial infection of lung
epithelial cells and production of viral Interleukin-8 (vIL-8) recruits the innate immune cells resulting in infection of macrophages and B-cells. By as early as 24 hr pi, the macrophages and dendritic cells can disseminate the virus from lungs to B cells and CD4+ T cells in the bursa of Fabricius, spleen, and thymus. During the cytolytic phase, large numbers of B-cells within the spleen along with CD4+ T cells in cecal tonsils undergo apoptosis and contribute to immunosuppression. A semi productive lytic viral replication in B cells and production of vIL-8 leads to recruitment and infection of T-cells which then leads to viremia and systemic spread of infection.
2) Immune evasion and latency phase: occurs between 7-10 dpi. MDV integrates into the genome of the infected CD4+ T cells leading to immune evasion and establishment of latency.
3) Cutaneous infection, replication and shedding: latently infected CD4+ T cells migrate to cutaneous feather follicles, where they infect the feather follicle epithelium. The resulting fully productive viral replication causes syncytia formation, and secretion of mature virions in skin dander. Aggregates of small lymphocytes with intranuclear inclusions can be seen in perifollicular areas by 7 dpi. These lymphoid aggregates develop into either cutaneous tumors or degenerate to form necrotic foci.
4) Proliferative phase: occurs around 28 dpi and is characterized by formation of CD4+ T cell visceral lymphoma. Meq (Marek´s EcoQ) gene has been shown to be important for transformation and Meq protein is consistently expressed in lymphoma cells and tumor cells5. The infection in transformed cells is nonproductive.
The two important and most common lymphomatotic diseases are Marek´s disease and lymphoid leukosis (ALV) differential diagnosis of the two is confusing. Table 2 describes the important features for their differential diagnosis.
|
Table 2: Gross and microscopic features for differential diagnosis of Marek´s disease and lymphoid leukosis1 |
||
|
Feature |
Marek´s disease |
Lymphoid leukosis |
|
Age |
Few days to many weeks |
Not less than 16 weeks |
|
Clinical signs |
Frequent paralysis |
Nonspecific |
|
Incidence |
Usually more than 5% in unvaccinated flocks |
Rarely more than 5% of infected flocks |
|
Gross lesions |
||
|
Neural enlargement |
Frequent |
Absent |
|
Bursa of Fabricius |
Diffuse enlargement or atrophy |
Nodular tumors |
|
Proventriculus, skin and muscle tumors |
May be present |
Usually absent |
|
Microscopic lesions |
||
|
Neural involvement |
Frequent |
Absent |
|
Cytology of tumors |
Usually pleomorphic lymphoid cells consisting of lymphoblasts, small, medium and large lymphocytes and reticulum cells |
Lymphoblasts of uniform morphology, usually of clonal origin |
|
Category of neoplastic lymphoid cell involved |
T lymphocyte |
B lymphocyte |
(Table adapted from Pattinson M et al., Poultry Diseases 2008.)
Marek´s disease virus is transmitted horizontally only, and appropriate hygiene precautions and vaccination can prevent its spread in hatching eggs and day-old chicks. ALV and REV can be transmitted both horizontally and vertically and avian leukosis and REV can be controlled by virus eradication at the primary breeding level.
Gross Pathology: The presence of enlarged peripheral nerves and/or visceral lymphomas are commonly seen in Marek´s disease, but these lesions are not pathognomonic9. Some of the commonly seen lesions include: 1) Enlargement of peripheral nerves (brachial, sciatic and coeliac plexus, abdominal vagus, and intercostal nerves) with loss of cross-striations and glistening appearance. The affected nerves are edematous and greyish or yellowish in appearance. 2) Development of lymphoid tumors in visceral organs of birds less than 16 weeks of age. 3) Discoloration of the iris and irregularity of the pupil. 4) Lymphomas in the skin around feather follicles.
Microscopic lesions: Affected nerves and visceral tumors contain mixed populations of small to large lymphocytes, lymphoblasts, plasma cells, and macrophages. The peripheral nerves affected in classical and acute forms show three types of lesions: A-type lesions (proliferative type) is characterized by infiltration of proliferating lymphoblasts, small to large lymphocytes, and macrophages; B-type lesion (inflammatory type) is characterized by edema and infiltration of small lymphocytes, plasma cells with proliferation of Schwann cells; C-type lesion (minor infiltrative type) is characterized by mild scattering of small lymphocytes and plasma cells, generally seen in birds with no clinical signs or gross lesions. The proportion of different cell types varies with stage of disease and virulence of the virus with aggressive lymphomas containing of a higher proportion of lymphoblasts.
Immunohistochemistry characteristics: MD tumor cells commonly express MHC-II and T cell markers such as CD4. 5-40% of the tumor cells express MATSA, less than 5% of the cells express IgM. Viral antigens pp384 and meq can be detected in tumor cells5 by immunohistochemistry, fluorescent antibody tests or in situ hybridization.10
Contributing Institution:
North Carolina State University College of Veterinary Medicine
https://cvm.ncsu.edu/research/departments/dphp/programs/pathology/
JPC Diagnosis: Liver: Lymphoma,
large T-cell.
JPC Comment: The contributor has done an excellent job in reviewing Marek´s disease, one of the most common neoplastic diseases of poultry (and unusual in the fact that it is caused by an alphaherpesvirus (avian alphaherpesvirus-2) rather than a gammaherpesvirus (lymphocryptovirus, rhadinovirus) which is more typical of herpesvirus-driven oncogenesis. )
Joszef Marek (1868-1952) was a noted Hungarian veterinarian, professor of pathology, and ultimately director of the veterinary school in Budapest. In 1907, he first described a peculiar neurological disease which he noticed in his backyard chickens, causing drooping of the wings, and paresis (and ultimately paralysis) of the legs. His multivolume textbook on animal pathology and therapeutics, written at the turn of the century with colleague F. Hutyra, was translated into numerous languages and enjoyed great popularity for decades. He discovered the use of ditrol to control liver flukes, and was awarded the Hungarian Kossuth prize for science in 1949.
Two reports (2016 and 2018) have also described Marek´s
disease in peafowl - one in the Indian peafowl (Pavo cristatus)2
more commonly known as the "peacock" and one in a green or Javan peafowl (Pavo
muticus)8. Both reports, which describe the pathologic
features as well as immunophenotyping of the neoplastic lymphocytes and
identification of Marek´s disease virus Serotype 1 by PCR, share a number of
similarities with this case, including the presence of disease in adult birds.2,8
The contributor lists a number of causes of lymphoproliferative disease in
poultry, including the common Marek´s disease and avian leukosis, the less
common avian reticulendotheliosis (a virus that may transform both B and T
cells in affected birds), and the relatively unknown retrovirus which causes
lymphoproliferative disease in turkeys (with a catchy syndromic name of
lymphoproliferative disease in turkeys.)1,6
LPDV virus is an exogenous retrovirus which causes lymphoid tumors in some galliforms, especially wild turkeys. The virus was identified first in Europe in the 1970´s, and not in North America until 2009.6 In a study of 800 wild turkeys over a 37 year period,6 lymphoid neoplasia was seen in approximately 7% of cases, and LPDV virus was identified in over half of those by PCR. In infected birds with neoplastic disease, skin tumors are most common (44%) with liver lesions in 30%. In a few birds in which the lymphocytes were immunophenotyped, the neoplasms appeared to be of T-cell origin, but additional information needs to be performed in this area.6
The moderator cautioned on the overinterpretation of histologic changes in a single HE slide to differentiated between Marek´s disease and lymphoid leukosis. While a positive immunohistochemical test for CD-3 is very helpful in further narrowing the potential diagnosis, definitive diagnosis is still best determined by real-time PCR (performed by the contributor in this case and positive.)
References:
1 Allison AB, Keel MK, Philips JE, Cartoceti AN, Munk BA, Nemeth NM, Welsh TI Thomas JM Crum JM et al. Avian oncogenesis induced by lymphoproliferative dissease virus: a neglected or emerging retrovirual pathogen Virology 2014; 450-451:2-12 doi: 10.1016/jvirol 2013.11.037. .
2 Blume GB, Cardoso SP, Oliveira MLB, Maziolli MP, Gomez SYM, Reis Junior JL, Sant Ana FIF, Martins NRS. Visceral Marek´s disease in white peafowl (Pavo cristatus). Arch Bras Med Vet Zootec 2016:68(6)1602-1608
3 Boodhoo N, Gurung A, Sharif S, Behboudi S: Marek´s disease in chickens: a review with focus on immunology. Veterinary Research 2016:47(1):119.
4 Gimeno IM, Witter RL, Hunt HD, Reddy SM, Lee LF, Silva RF: The pp38 Gene of Marek´s Disease Virus (MDV) Is Necessary for Cytolytic Infection of B Cells and Maintenance of the Transformed State but Not for Cytolytic Infection of the Feather Follicle Epithelium and Horizontal Spread of MDV. Journal of Virology 2005:79(7):4545-4549.
5 Nair V: Latency
and tumorigenesis in Marek´s disease. Avian Dis 2013:57(2 Suppl):360-365.
6. Neidringhaus,KD, Nemeth NM, Sellers HS, Brown JD, Fenton HMA. Multicentric round cell neoplasms and their virsal associataions in wild turkeys (Melagris gallopavo) in the southeastern United States. Vet Pathol 2019; doi 10.1177/0300985819864306
7. Pattison PM, Bradbury J, Alexander D. eds., In: Poultry Diseases. 6th ed.: Saunders Ltd.; 2008: 258-275.
8. Ranjbar VR, Khordadmehr R. Marke's disease in a peafowl (Pavo muticus); pathological, immunohistochemical and molecular studies. Conference proceedings. Appr Poultr Dairy Vet Sci: 2018.
9 Seimon TA, McAloose D, Raphael B, Honkavuori KS, Chang T, Hirschberg D, et al.: A novel herpesvirus in three species of pheasants: Mountain peacock pheasant (Polyplectron inopinatum), Malayan peacock pheasant (Polyplectron malacense), and Congo peafowl (Afropavo congensis). Veterinary pathology 2012:49(3):482-491.
10. Swayne DE, ed. Neoplastic Diseases. Diseases of Poultry. 13 ed.; 2017.
11. Witter RL: Increased virulence of Marek´s disease virus field isolates. Avian Dis 1997:41(1):149-163.