Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 3
Sept 12, 2018
CASE I: AFIP 15-13332 (JPC 4067411-00).
Signalment: 3-week-old male domestic shorthair kitten
History: A 3-week-old male domestic short hair kitten was one of 8 kittens found before taken to a cat rescue. The litter was split into two different foster homes. All kittens developed diarrhea with lethargy and inappetence. The kitten in this case was euthanized after a week of developing symptoms and two more died subsequently (exact timing unknown). The remaining two surviving kittens are alive and thriving.
Gross Pathology: A male kitten (0.11 kg) in fair body and postmortem condition was presented for autopsy. The umbilicus has no lesions. The stomach is expanded by ~ 5 ml of curdled milk. The rectum and distal colon contain yellow-green dry string-like fecal material. Both lungs are wet and mottled dark and pale pink with areas of hyperinflation. No other significant gross lesions identified.
Laboratory results: Hemolytic E. coli and group G beta-hemolytic Streptococcus were both cultured from pulmonary tissue.
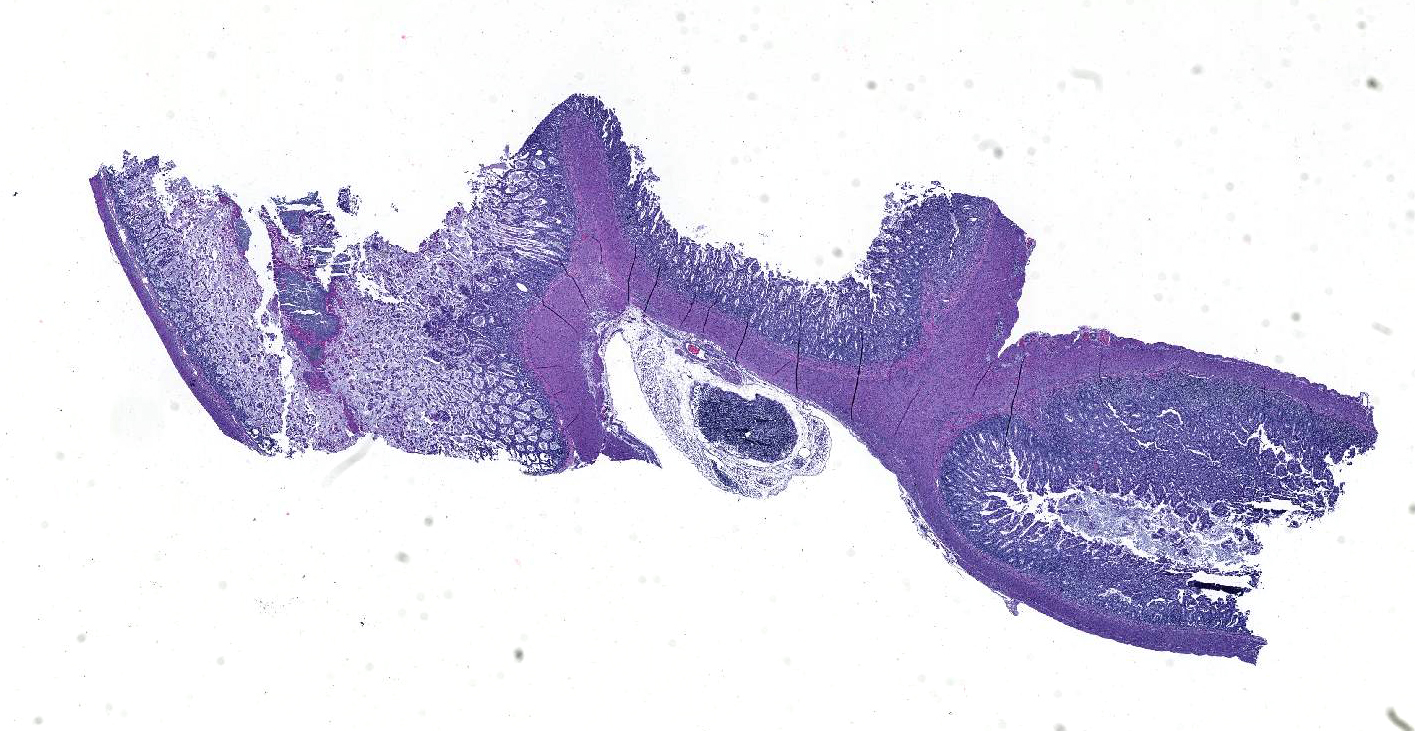
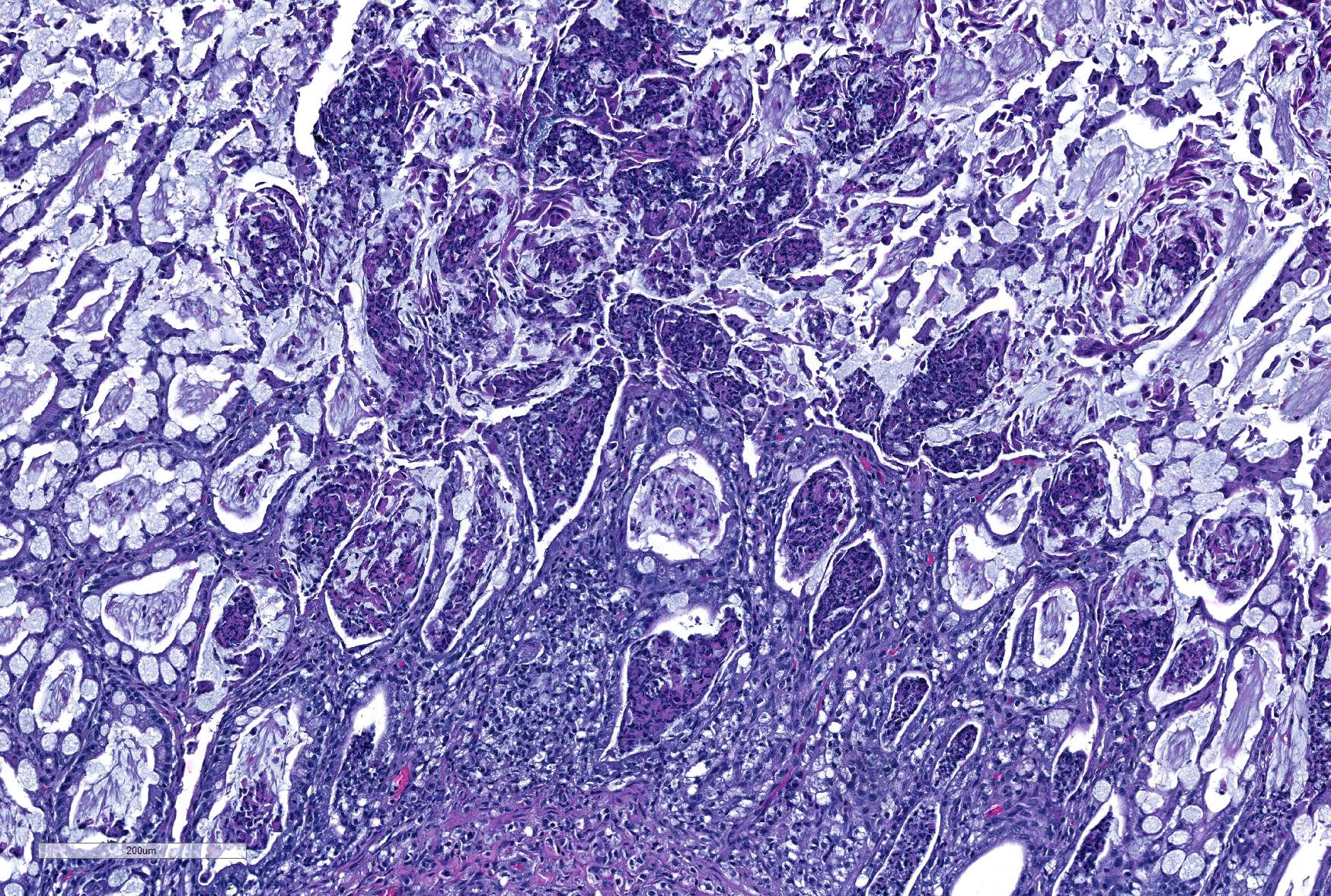
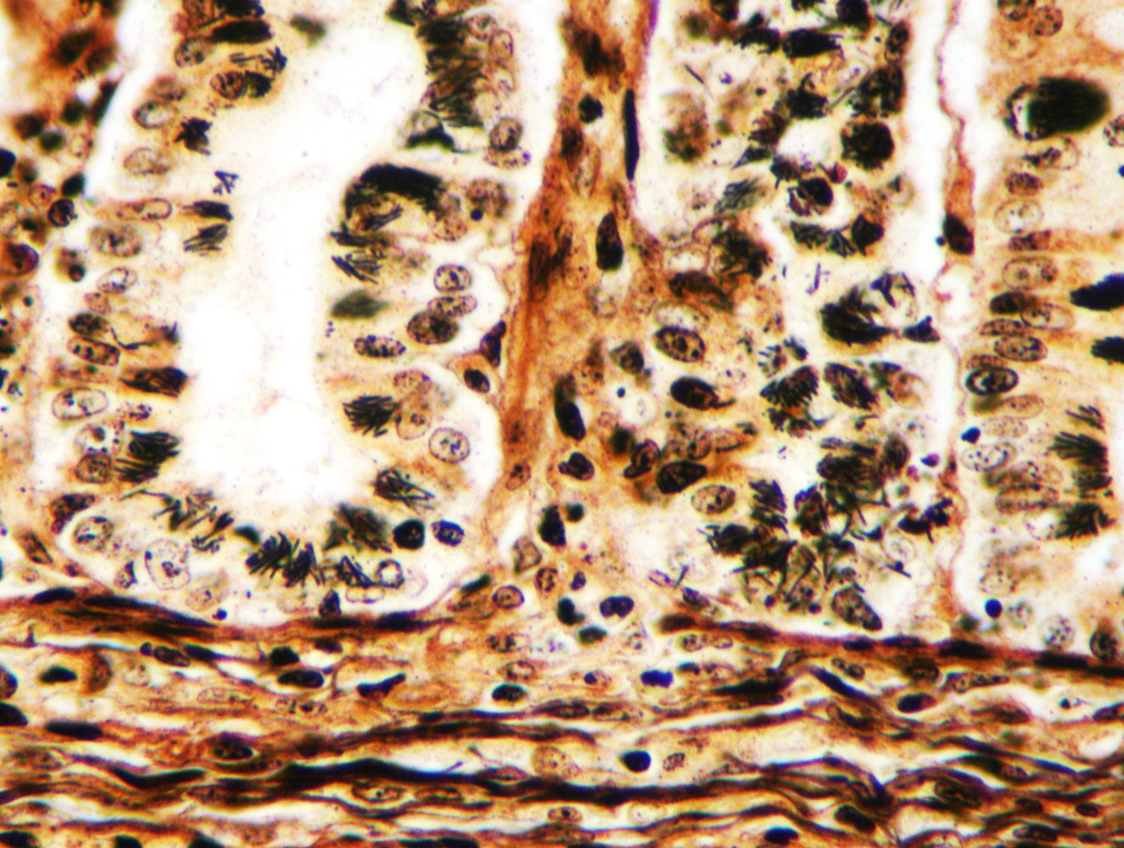
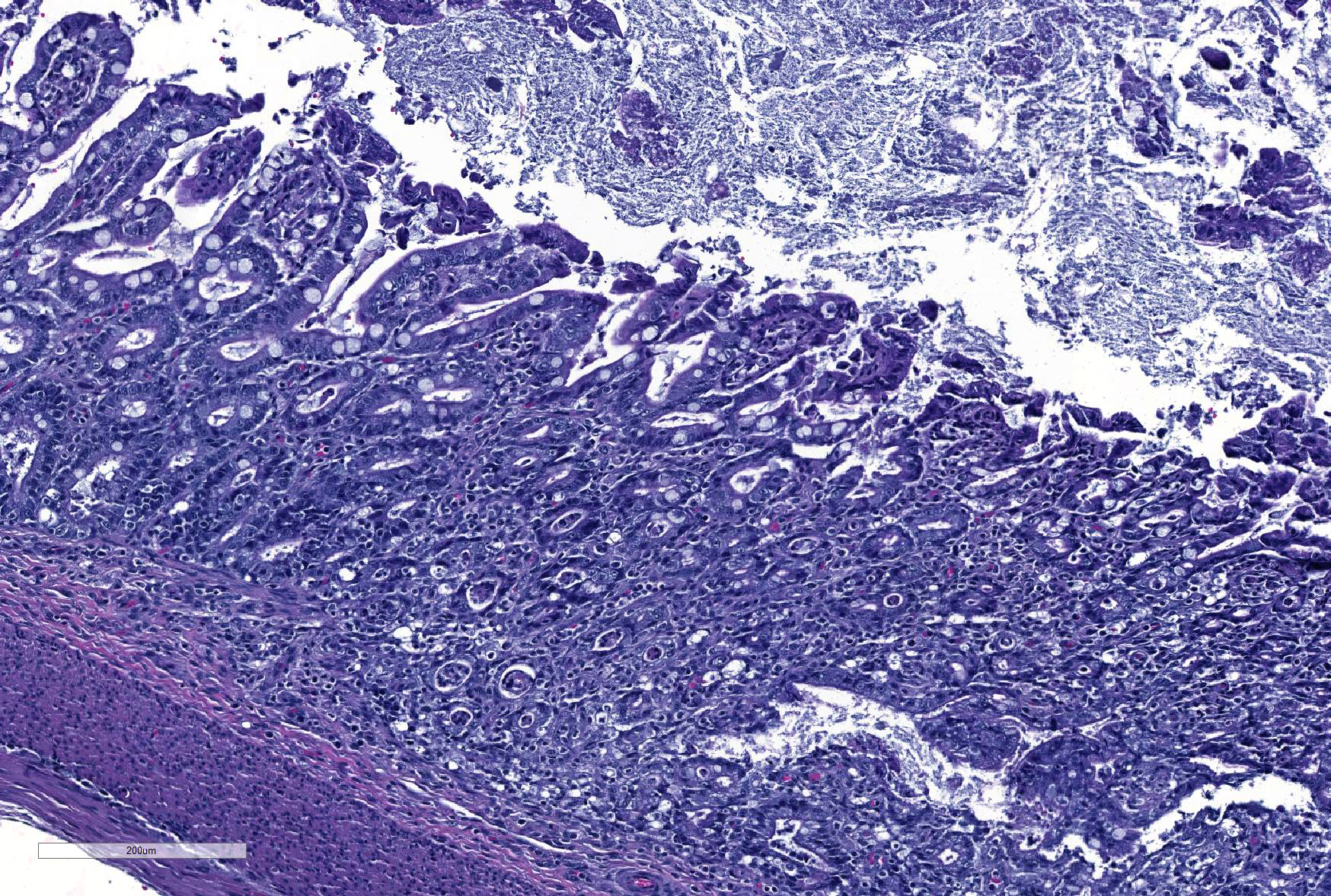
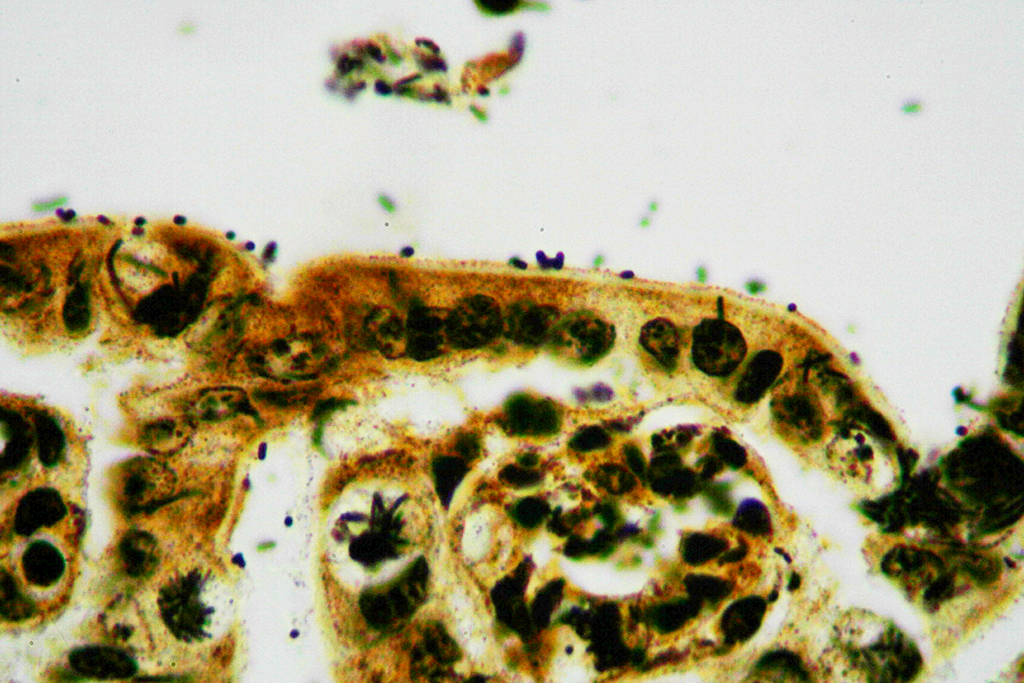
Microscopic Description: Ileum and colon. The section of ileum contains frequent crypt necrosis and ~50% intestinal villi blunted with occasional complete villous loss. There are large numbers of cocci and rod bacteria within the lumen and crypts of the tunica mucosa, both extracellularly and intracellularly, throughout the majority of stained sections. Slender rod-shaped bacteria are arranged in one or more parallel stacks within the cytoplasm of numerous enterocytes. The lumen of the ileum is heavily colonized by a mixed population of gram-negative rods and cocci, with moderate numbers of cocci directly associated with the surface of mucosal epithelial cells. The tunica muscularis and serosa appear unaffected. The section of intact colon is included to demonstrate that the changes to the ileum are not likely due to autolysis. The colon contains few luminal bacteria, but moderate numbers of enterocytes contain slender rod bacteria as described above.
Contributor’s Morphologic Diagnoses: Ileum and colon: Marked multifocal to coalescing necrotizing enterocolitis with extracellular, adherent and intracellular mixed bacteria.
Contributor’s Comment: Differentials to consider in a case of enterocolitis in neonatal kittens include: bacteria (E. coli, Salmonella), primary viral enteritis (parvovirus, enteric coronavirus), protozoa (Isospora, Giardia) or severe systemic disease, such as caused by feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV). The two surviving kittens were 8 weeksold at the time of this submission and both had tested negative for FIV and FeLV the week prior. Culture of fecal samples from both kittens yielded E. coli, however only one harbored the hemolytic phenotype. Salmonellae were not recovered using routine media.
In the case presented here, there are concurrent intestinal bacterial infections by C. piliforme and E. coli. E.coli had primarily colonized the small intestine while C. piliforme was associated with crypt necrosis in the large intestine. An area of overlap within the ileum, as highlighted in the submitted slide, shows the co-existence of both bacteria. There have been few published feline cases of Tyzzer's disease, and a bacterial co-infection in a cat has not yet been reported. A recent publication, however, describes dual infection by C. piliforme and enteropathic and effacing E. coli causing enterotyphlocolitis in a Syrian hamster.1
Tyzzer’s disease in cats has been associated with viral co-infection with FIP and FeLV.2,5 Coronaviral protein was not detected in the small intestine or colon by immunohistochemistry in this kitten (a quantity of fecal material sufficient for testing could not be obtained during necropsy); thus, a concurrent or underlying and predisposing viral infection, while unlikely, cannot be completely ruled out. Other factors that are more likely to have predisposed this kitten to Tyzzer’s disease are failure of passive transfer, environmental stress (exposure, relocation), immune compromise, or concurrent disease.
Suboptimal immune competency of the host has been associated with the dissemination of extraintestinal pathogenic E. coli.4 Colonization of the small intestine with E. coli and subsequent sepsis could have been a primary event in this case. Bacterial culture identified moderate numbers of hemolytic E. coli and low numbers of group G beta-hemolytic Streptococcus in the lungs. Histopathology of the lung identified mild, diffuse, interstitial pneumonia consistent with sepsis.
- coli is a constituent of the normal feline intestinal microflora and does not normally produce disease. When provided with a compliment of virulence factors, however, E. coli may become an opportunistic pathogen resulting in local and systemic infection and even death. At least seven distinct pathotypes have been identified, including enteropathogenic (EPEC), enterotoxigenic (ETEC), and shiga-toxin producing E. coli (STEC; also referred to as enterohemorrhagic E. coli or EHEC).8 Shiga toxin-producing Escherichia coli (STEC) causes hemolytic uremic syndrome (HUS) in humans and has zoonotic potential. Most shiga-toxin producing E. coli belong to various serotypes including O26, O103, O111, O145, and O157, the latter being the most commonly identified.7 A study of cats and dogs in Buenos Aires, Argentina, where STEC is an endemic pathogen, identified O157 in 4/149 (2.7%) cats and 34/450 (7.5%) dogs (3). Testing of the E. coli isolate did not detect O157:H7 antigen in this case.
Group G beta-hemolytic Streptococcus isolates include: Streptococcus dysgalactiae subspecies equisimilis, S. milleri, S. canis, and S. intestinalis. Both S. dysgalactiae subspecies equisimilis and S. milleri are associated with human infection, while dogs and pigs are reservoirs for S. canis and S. intestinalis, respectively. S canis is the most likely strain to be identified in this case and is typically considered a commensal and extracellular pathogen in cats, however it has been shown to cause severe feline disease such as necrotizing fasciitis, sinusitis, bacteremia, and toxic shock–like syndrome.9 Because of the low numbers of Streptococcus organisms isolated from the lungs, and absence of pathogenic changes consistent with primary Streptococcus infection, we consider this an opportunistic colonization in this cat.
The clinical presentation of Tyzzer’s disease can range from subclinical infection and incidental finding to mild to marked mucoid diarrhea or death without premonitory signs. The mechanism of host cell attachment and entry by C. piliforme is not fully understood. Following colonization of the distal ileum, cecum, and colon, bacteria can penetrate into the portal vasculature to seed the liver.7 Neither necrotizing inflammation nor clostridial forms were identified within the liver in this kitten. C. piliforme infection limited to the intestinal tract could reflect an early stage of infection, prior to hematogenous spread, and would suggest that C. piliforme colonization occurred secondary to E. coli infection.
JPC Diagnosis: 1. Colon: Colitis, necrotizing, diffuse, marked with numerous crypt abscesses, crypt hyperplasia and intracytoplasmic bacilli within crypt epithelium.
2. Ileum: Ileitis, necrotizing, diffuse, mild to moderate with numerous surface-adherent bacilli.
Conference Comment:
Ernest Edward Tyzzer (1895-1965) was an American pathologist whose investigations far exceeded the disease which today bears his name. A graduate of Harvard medical school in 1902, many of his discoveries impacted veterinary science far more than human medicine. In 1901, he received a master’s degree studying myxosporidian infections in menhaden. Between 1905 and 1916, he was research director for the Harvard Cancer Commission, investigating spontaneous and transplanted tumors in mice, but continuing his interest in infectious diseases as well.10 In 1910, he identified the genus Cryptosporidium sp. for the first time. He was responsible for the development of the DBA inbred strain of mouse during this time, as well as the identification of Leishmania sp. as the cause of Ooroya fever. 10
In 1916, Dr. Tyzzer became the head of the Department of Comparative Pathology at Harvard, a chair he held until his retirement in 1942.10 In 1917, he first identified the disease that now bears his name in Japanese waltzing mice, a strain he was utilizing in cancer research. In this position, he correctly identified the flagellate Histomonas meleagridis as the causative agent of blackhead, as well as detailing its life cycle. (The principles which he used to raise turkeys on his experimental farm were subsequently published by the Massachusetts Department of Agriculture and he was given a citation by the Governor of Massachusetts for “saving the turkey industry of Massachusetts).10 Additional investigations during his career provided seminal work in equine encephalomyelitis as well as coccidiosis in gallinaceous birds.
Over a century following its identification, many questions remain about C. piliforme. A common commensal in rodents, disease has been difficult to reproduce in healthy dogs and cats. Concomitant disease, especially infection with immunosuppressive viruses such as FELV, FIV, feline panleukopenia, and the mutated coronavirus of feline infectious peritonitis have been incriminated in the majority of feline infections, and experimental immunosuppression by glucocorticoids or cyclophosphamide has also been used to enhance experimental infections.6 Transmission from the feces of rodents has been suggested as a source of infection of dogs and cats, but has not been proven. Basic information about the pathogenesis of C. piliforme infection, such as the mechanisms of attachment and entry into cells by C. piliforme, have not yet been identified, and inability to culture the organism on artificial media further hampers laboratory research.6
Attaching and effacing E. coli (AEEC), which has been reported in a wide variety of species (and is the only pathotype reported in the rabbit), employs a unique procedure for colonization of the intestine. The classic “cup and pedestal” attachment to the luminal surface results from the bacteria’s ability to manipulate the host cytoskeleton, in particular the recruitment of actin filaments beneath adherent bacteria.8 An additional virulence factor of AEEC is intimin and its receptor-translocated intimin receptor (TIR). TIR is secreted directly by AEEC into the cytoplasm of intestinal epithelium via injectisomes (aka, needle complexes or T3SS apparatus), whereupon intimin expressed on the bacterial surface.8
Conference participants reviewed the pathogenesis of Tyzzer’s disease in a variety of animal species, as well as reviewed the various common pathotypes of E. coli which produce disease in domestic species. A Giemsa stain run on this slide demonstrated both the mucosal-adherent E.coli as well as the intracellular C. pilforme extremely well.
Contributing Institution:
Oregon State University Diagnostic Laboratory
http://vetmed.oregonstate.edu/diagnostic
References:
- Aboellail TA, Hk N, Mahapatra D. A Naturally Occurring Enterotyphlocolitis Associated with Dual Infection by Clostridium piliforme and Enteropathogenic Attaching and Effacing Escherichia coli in Syrian Hamsters. J Vet Sci Med 2013;1.
- Bennett AM, Huxtable CR, Love D. Tyzzer’s Disease in Cats Experimentally Infected with Feline Leukaemia Virua. Vet Microbiol 1977;2:49–56.
- Bentancor A, Rumi MV, Gentilini MV, Sardoy C, Irino K, Agostini A et al. Shiga toxin-producing and attaching and effacing Escherichia coli in cats and dogs in a high hemolytic uremic syndrome incidence region in Argentina. FEMS Microbiol Lett 2007;267:251–256.
- Bleibtreu A, Gros P-A, Laouénan C, Clermont O, Le Nagard H, Picard B et al. Fitness, stress resistance, and extraintestinal virulence in Escherichia coli. Infect Immun 2013;81:2733–2742.
- Ikegami T, Shirota K, Goto K, Takakura A, Itoh T, Kawamura S et al. Enterocolitis associated with dual infection by Clostridium piliforme and feline panleukopenia virus in three kittens. Vet Pathol 1999;36:613–615.
- Jones BR, Greene CE. Tyzzers Disease. In Greene, CE, ed. Infectious Diseases of the Dog and Cat, 4th edition. St. Louis, MO. Elsevier Saunders, 2012 pp. 391393.
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran’s Pathologic Basis of Disease, Professional Edition: Expert Consult-Online.
- Marks SL, Rankin SC, Byrne BA, Weese JS. Enteropathogenic Bacteria in Dogs and Cats: Diagnosis, Epidemiology, Treatment, and Control. J Vet Intern Med 2011;25:1195–1208.
- Pesavento PA, Bannasch MJ, Bachmann R, Byrne BA, Hurley KF. Fatal Streptococcus canis infections in intensively housed shelter cats. Vet Pathol 2007;44:218–221.
- Weller TH. Ernest Edward Tyzzer 1875-1965, A biographical memoir. Nat Acad Sci 1978; 49(14):351-373.