Signalment:
16-year-old, male, bar-headed goose, (
Anser indicus).Between
September 9 and September 17, 2015, three bar-headed geese housed in an open,
outdoor enclosure near Village Lagoon died unexpectedly. The first two had been
found dead on exhibit, while the third had been hospitalized shortly before
death with clinical signs of labored breathing and lethargy. The hospitalized
goose had a CBC within normal limits. At the same time, a fourth bar-headed
goose was hospitalized with diarrhea.
Gross Description:
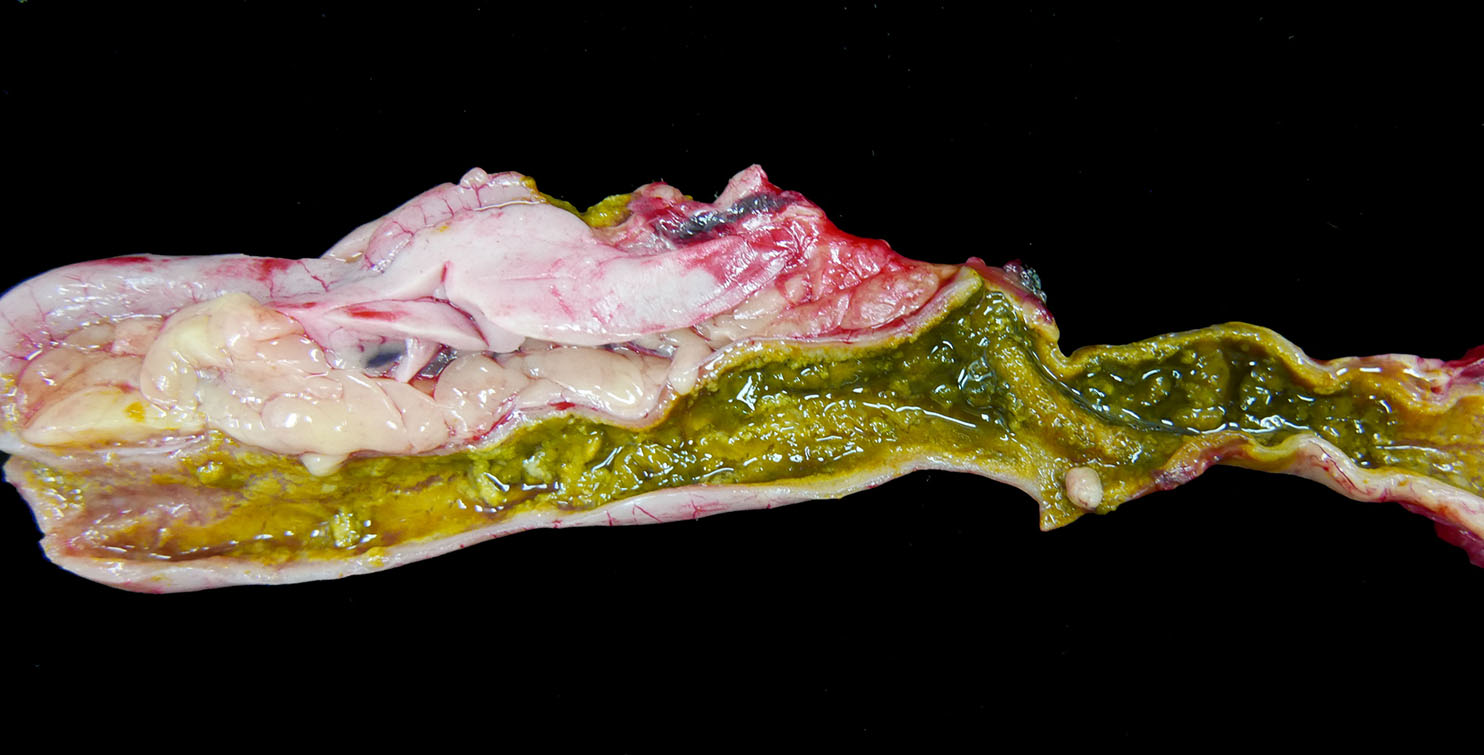
The
spleen has a few ill-defined, pale tan foci of necrosis extending from
the serosa into the parenchyma. The pancreas has 10 to 15 scattered, tan,
well-circumscribed necrotic foci measuring up to 0.2 cm in diameter. The
entire small and large intestine are filled with tan to green, fibrous, clumped
soft material that coats the mucosa in some regions and sloughs away along
other portions. Within the ceca, there is a small to moderate amount of
tan-green watery fluid and tan fibrous strands that loosely adhere to the
mucosal surface.
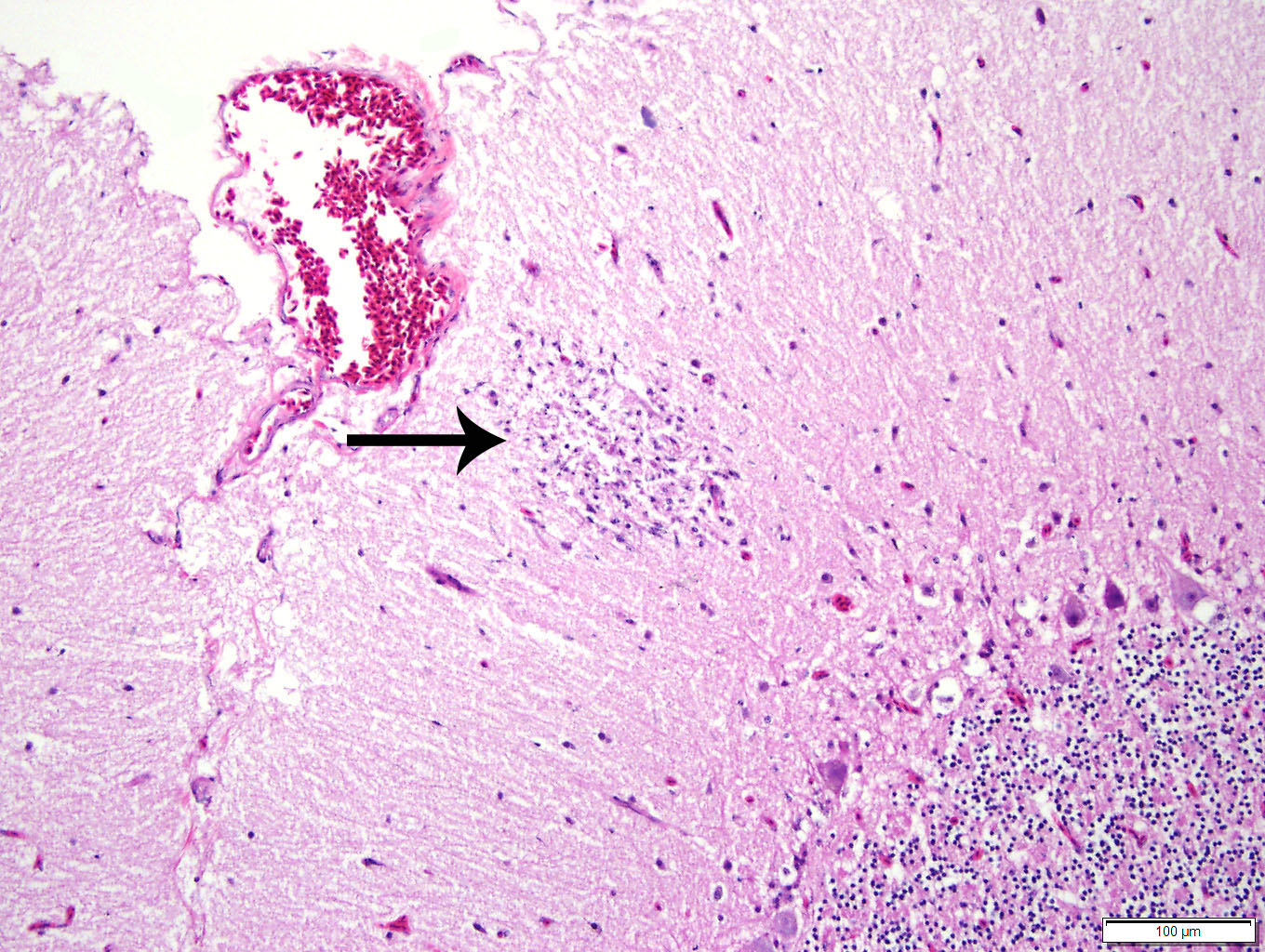
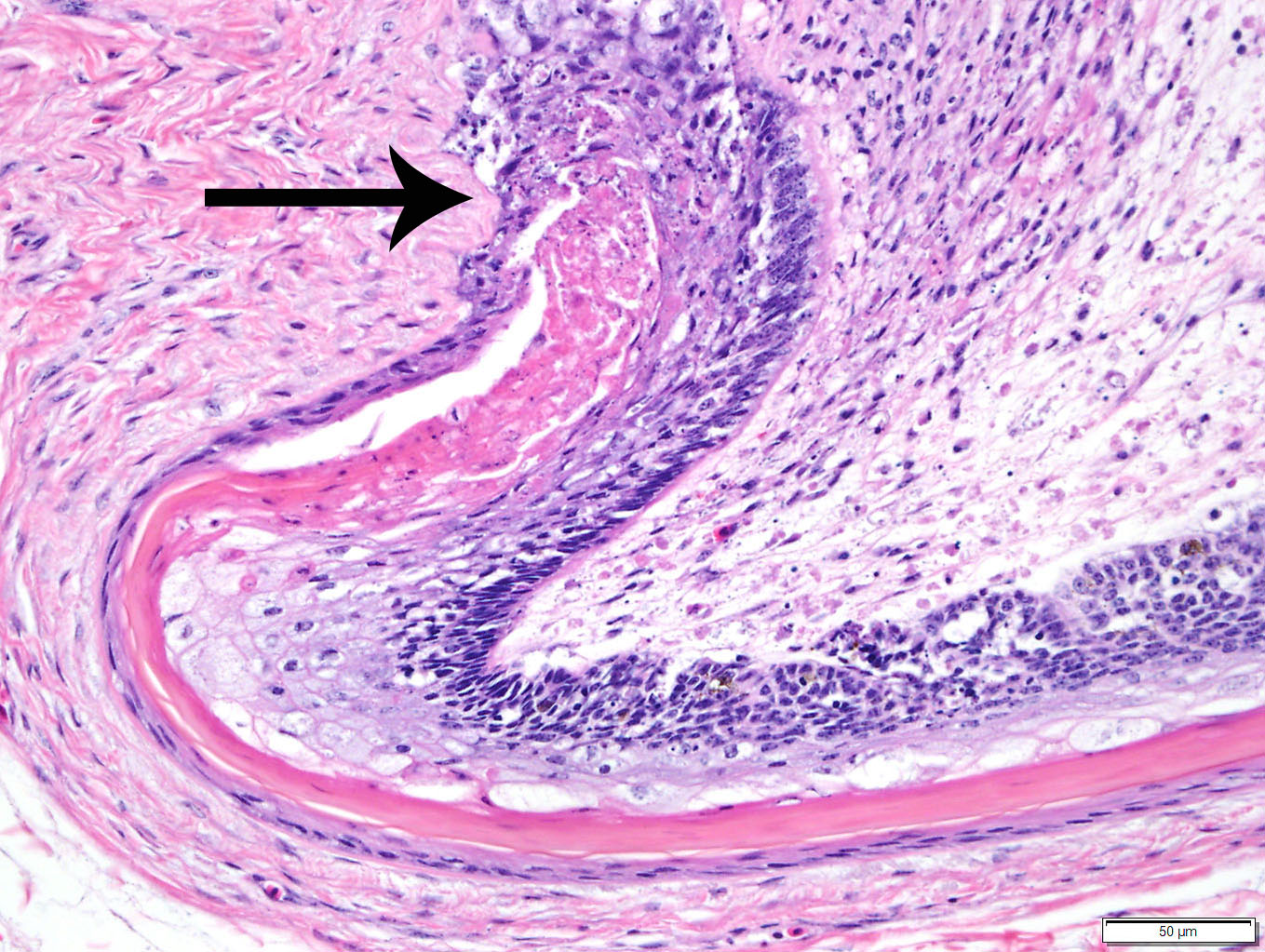
Histopathologic Description:
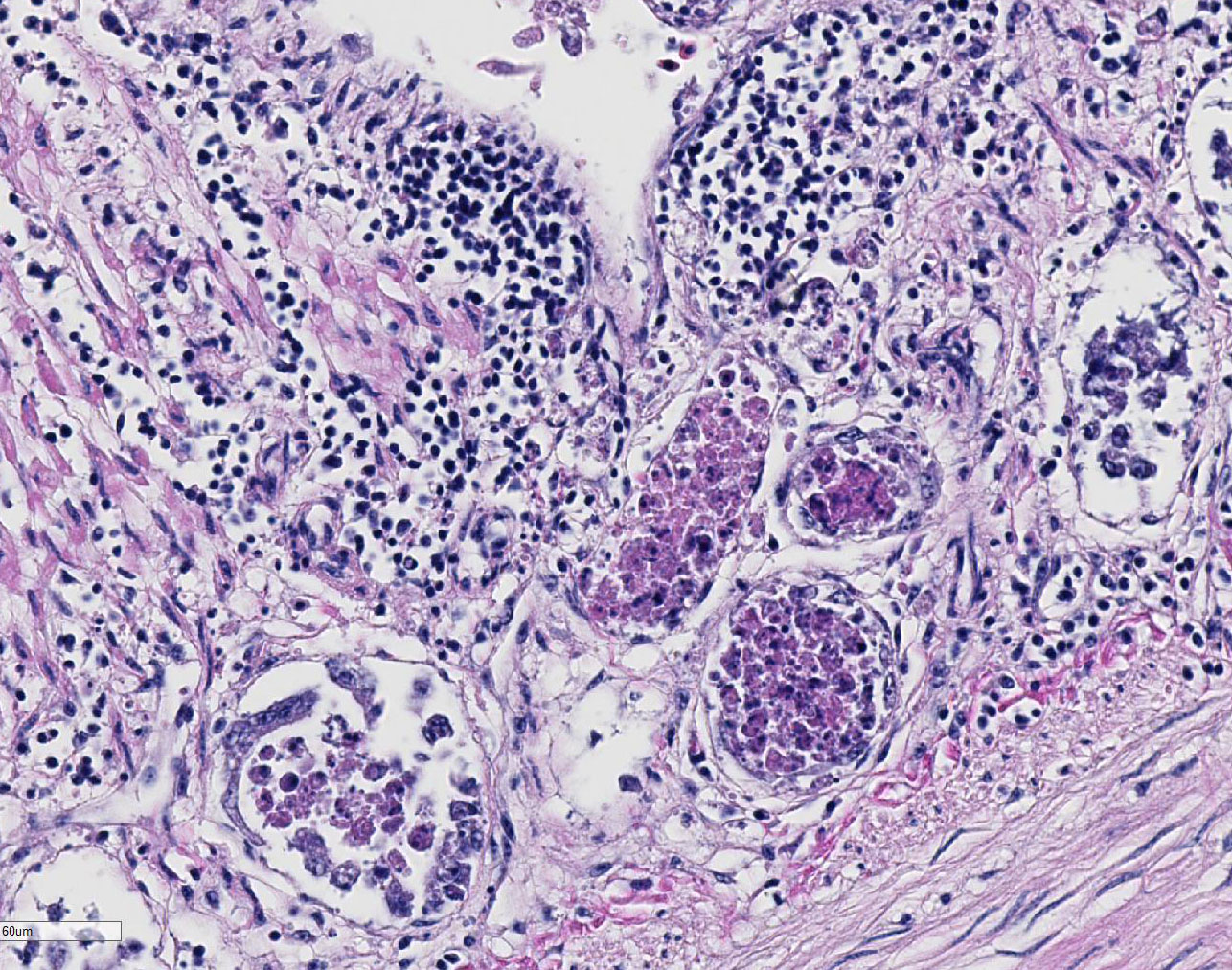
Duodenum
and pancreas: Sections are mildly autolyzed. In the duodenum, villi are
markedly blunted and frequently fragmented. The majority of crypts are dilated
with hypereosinophilic, karyorrhectic cellular debris (crypt abscesses), and
glandular epithelial cells are frequently shrunken and hypereosinophilic with
nuclear pyknosis (necrotic). The villous epithelium is sloughed and is
regionally replaced by luminal bands of necrotic sloughed cells, degenerate
heterophils, and large numbers of embedded bacteria (pseudomembrane). Moderate
to large numbers of lymphocytes with fewer plasma cells populate the lamina
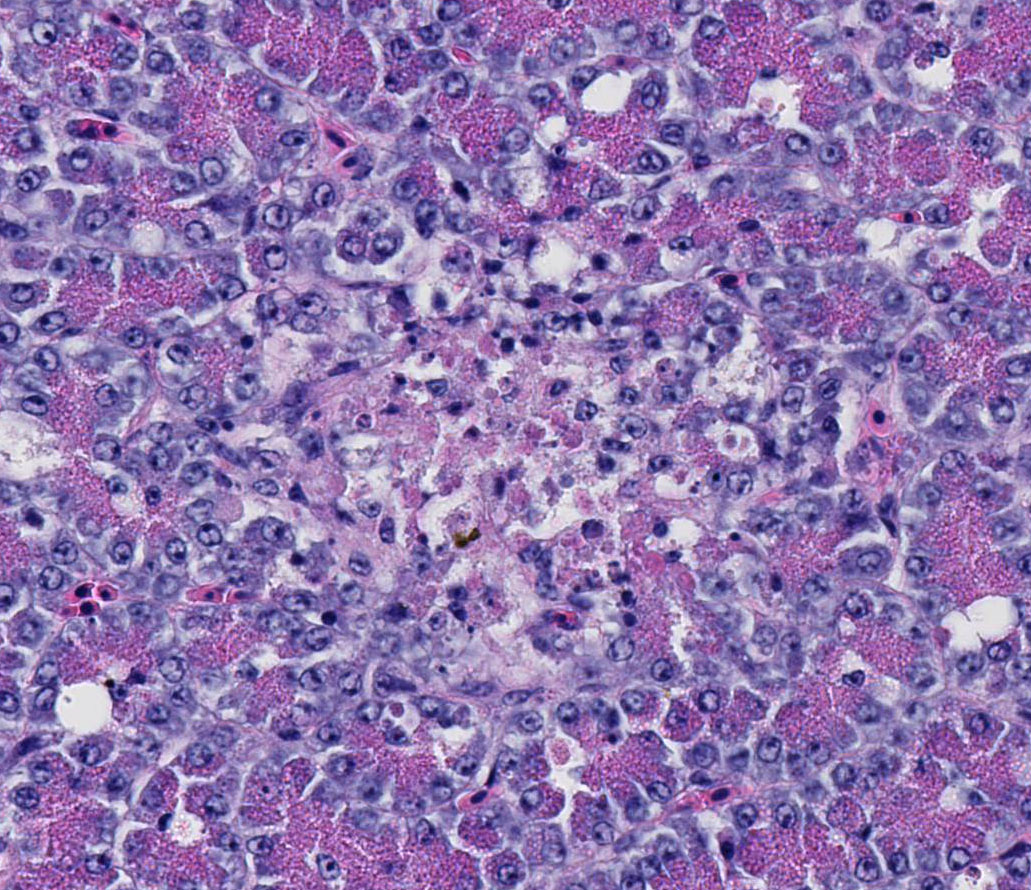
propria. Throughout the exocrine pancreas, there are multiple, randomly
distributed foci of necrosis, characterized by hypereosinophilia, loss of
cellular detail and karyorrhexis, and small numbers of heterophils. Foci of
necrosis are often surrounded by a band of acinar cells with marked zymogen
depletion. There are occasional
ill-defined regions of exocrine pancreatic hyperplasia, with lobules of tightly
packed, slightly haphazardly arranged acinar cells delineated by coarse fibrous
septa.
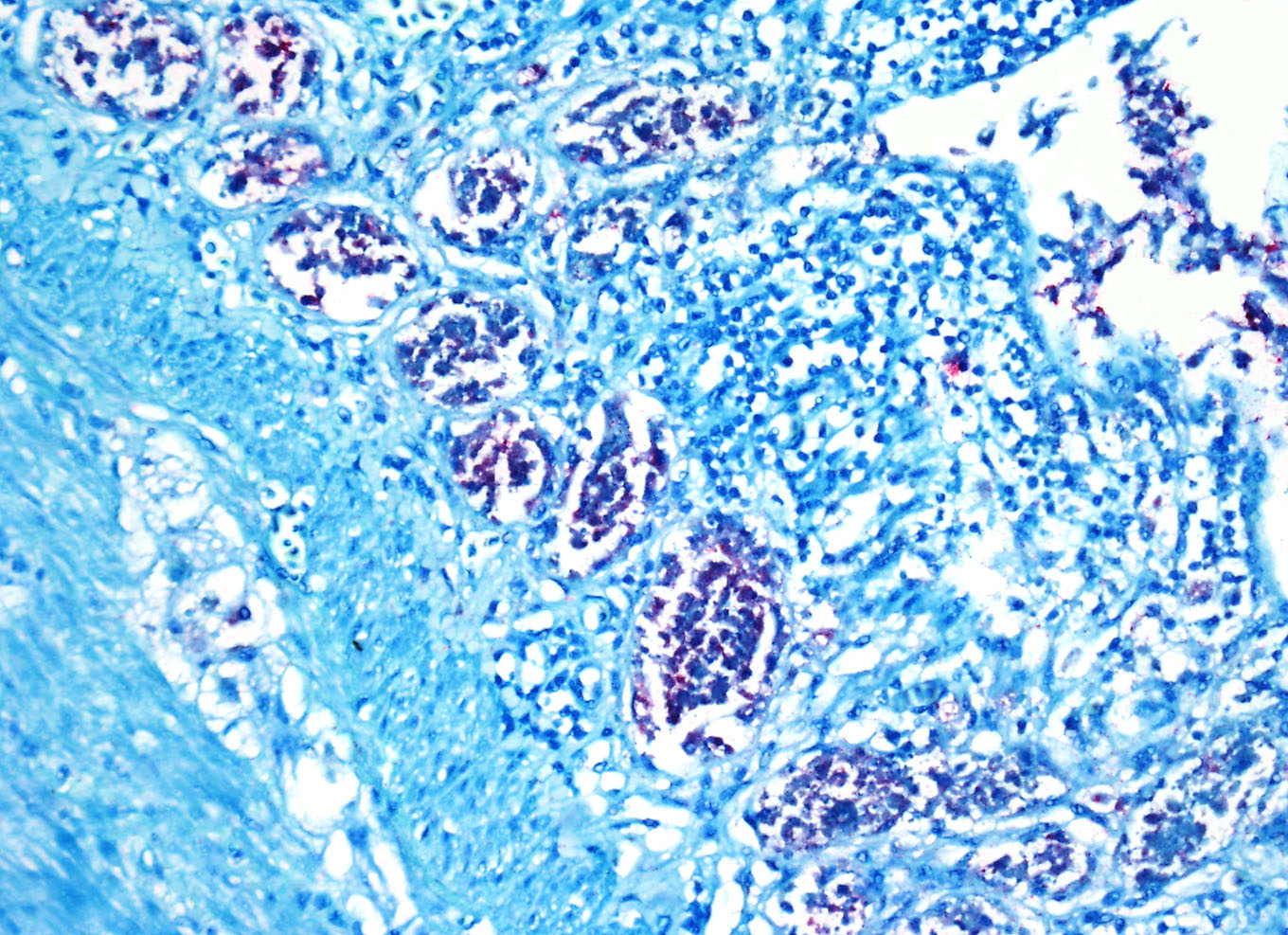
Immunohistochemistry
for West Nile Virus (performed in-house): Strong positive cytoplasmic
immunoreactivity within intestinal epithelial cells or foci of crypt necrosis,
pancreatic acinar cells, glial nodules in the brain, renal tubular epithelial
cells, cardiomyocytes, hepatocytes, and in the spleen (macrophages).
Morphologic Diagnosis:
1. Duodenum: Severe, diffuse, acute, necrotizing
enteritis with segmental pseudo-membranes and mixed bacteria
2. Pancreas:
Moderate, multifocal, acute necrosis
Lab Results:
PCR
and virus isolation were performed by the California Animal Health and Food
Safety diagnostic laboratory (CAHFS).
West Nile Virus
(avian) PCR: Virus detected in brain
Virus isolation:
West Nile Virus
Avian Influenza
Matrix Gene qRT-PCR: Not detected in lung, duodenum, or spleen.
Avian
Paramyxovirus-1 qRT-PCR: Not detected in lung, duodenum, or spleen.
Bacterial
culture, colon contents (IDEXX): 3+
Escherichia coli, 1+
Aeromonas
species, 2+ Normal positive flora. No
Salmonella, Shigella,
Pleisiomonas, Edwardseilla, Aeromonas or
Yersinia were isolated.
Condition:
Intraerythrocytic protozoan trophozoites/Babesia bovis
Contributor Comment:
Necropsy
and histopathology findings on all three bar-headed geese (
Anser indicus)
were consistent with severe systemic viral infection, with acute necrosis
most severely affecting the gastrointestinal tract, but also including
pancreas, trachea, spleen, esophagus, feather follicle epithelium and brain.
The three mortalities within a short time span, in conjunction with the
spectrum of histologic lesions (widespread tissue necrosis), met internal
criteria requiring notification of government regulatory officials (California
Department of Food and Agriculture, CDFA) and testing to rule out
highly-pathogenic avian influenza (HPAI). Immediate biosecurity measures were
implemented. Suspicion of HPAI was heightened by the fact that bar-headed geese
were the migratory waterfowl species predominantly affected in H5N1 HPAI virus
epidemics in China in 2005 and 2006.
6,9 Within approximately 48
hours of notifying CDFA, we received negative test results for HPAI, and CDFA
lifted the quarantine. Concurrently, West Nile virus (WNV) infection in all
three geese was confirmed by in-house immunohistochemistry. These results were
corroborated by PCR and virus isolation performed by the California Animal
Health and Food Safety diagnostic laboratory (CAHFS). The presence of such
striking, acute intestinal crypt necrosis was considered to be unusual for WNV
in an avian species, especially with only minimal brain lesions and an absence
of myocardial lesions.
West Nile Virus
is in the genus
Flavivirus, family
Flaviviridae, and is
serologically classified within the Japanese encephalitis antigenic group. The
virus is distributed worldwide and has a wide potential host range, but is
maintained primarily in a bird-mosquito cycle. Wild birds (especially corvids)
act as amplifying hosts.
Culex spp mosquitoes are the primary vector,
although the virus is found in other vectors (other mosquito species, ticks) of
undetermined significance in transmission.
3,5 Horizontal
transmission
2 and transmission through prey or contaminated water
have also been reported.
5 There are seven genetic lineages of WNV
strains, with two major lineages, lineage 1 and lineage 2. WNV genetic lineage
1 is widespread geographically, including in North America. Lineage 2 WNV
strains correspond primarily to enzootic areas in Africa, with recent detection
in Europe. Lineage 1 strains have been considered more virulent, but both have
been implicated in significant disease outbreaks
in birds.
3,5 Mortality due to WNV has been documented in 24 orders
of birds from North America, including anseriforms.
5
In mammals,
after a bite by an infected mosquito, the virus replicates in keratinocytes,
cutaneous dendritic cells, endothelial cells, and fibroblasts, followed by
viremia and hematogenous spread.
3 In avian species, the mechanism
and sites of replication are not completely understood. Experimentally in
birds, virus has been detected in blood within 45 minutes of mosquito biting,
suggesting that primary viremia can occur without local replication.
5
Clinical disease develops upon viral invasion of major organs and/or the CNS,
usually by 5-6 days post-infection.
7 In most infected birds, virus
is detectable first in the spleen, followed by spread to other visceral organs,
and then to the CNS.
5 Lesions of WNV in birds are often more
extensive in less susceptible species, such as chickens, with multi-organ
failure, peracute death and few to no lesions in highly susceptible species
like corvids. Chronic, persistent WNV infections have also been documented in
some species of birds, including house finches (
Haemorhous mexicanus)
and Western scrub-jays (
Aphelocoma californica).
7
In most orders
of birds, histologic lesions of WNV are primarily found in the CNS, heart,
liver, kidney, and spleen. Typical lesions in the CNS include mononuclear
meningo-encephalitis with perivascular cuffing, gliosis, and glial nodules. In
other organs, lesions are characterized by lympho-plasmacytic and histiocytic
inflammation, accompanied by cellular degeneration or necrosis. While the
intestinal lesions seen in the present case, with striking enterocyte and crypt
necrosis, have been described in WNV-infected corvids and other passerines,
they have not been described in Anseriformes.
1,2, 4,5, 8 Reports of
naturally and experimentally infected geese of various species have emphasized
pronounced myocardial lesions and moderate to severe mononuclear meningoencephalitis.
1,2,
4, 8
JPC Diagnosis:
1.
Small intestine: Enteritis, necrotizing, diffuse, severe
with crypt abscesses, bar-headed goose,
Anser indicus.
2.
Pancreas: Pancreatitis, necrotizing, random, multifocal, mild.
Conference Comment:
This excellent case
demonstrates the widespread tissue tropism that West Nile virus (WNV) has in
avian species. Most wild birds infected with WNV have a prolonged viremia
allowing dissemination of the virus throughout the body, affecting nearly every
organ. Typically, microscopic lesions associated with WNV viremia are
lymphoplasmacytic and histiocytic inflammation with degeneration and necrosis
within the central nervous system (CNS), heart, spleen, kidney, and liver. The
virus is distributed throughout the world and outbreaks can cause acute death
in a variety of different avian species. Highly susceptible birds include
crows, jays, and magpies may die so acutely that there are little to no
macroscopic lesions. Chronic disease, characterized by severe inflammatory
lesions, emaciation,
dehydration, hemorrhage, and congestion, occurs in avian species with
lower susceptibility to WNV infection such as owls, hawks, and psittacine
birds. Choroiditis, iridocyclitis, and retinal necrosis leading to progressive
visual impairment and blindness are reported in naturally WNV infected
red-tailed hawks and experimentally infected partridges and pheasants.
As mentioned by
the contributor, the virus is transmitted predominantly by
Culex sp.
mosquitoes. Corvid birds, such as the American crow, are the main amplifying
host and the virus is maintained in a bird-mosquito-bird lifecycle. Despite being
dead end hosts, a variety of mammalian species can be infected, with humans and
horses particularly susceptible to developing clinical disease. Transmission
occurs during the late spring to early fall during favorable weather conditions
for the mosquito vector. In contrast to avian species, lesions in horses are
confined to the central nervous system, primarily within the grey matter of the
brainstem and thoracolumbar spinal cord and less commonly in the cerebrum and
cervical spinal cord. Histologic lesions are typically lymphoplasmacytic
meningo-encephalomyelitis with glial nodules, lymphohistiocytic perivascular
cuffing, ring hemorrhages, neuronal degeneration, and necrosis. The preferred
modality for postmortem diagnosis of WNV infection in mammals includes
histologic identification of the previously mentioned lesions, in addition to
polymerase chain reaction (PCR) testing of the brainstem for WNV antigens. Even
in severe equine cases, viral antigen is typically scant within the central
nervous system making immunohistochemistry (IHC) in-situ hybridization (ISH)
less useful in horses.
Ruminants, dogs,
cats, and pigs are susceptible to infection, but usually only have transient
and subclinical disease; however, a recent report in
Veterinary Pathology
describes severe lympho-plasmacytic meningoencephalitis in six WNV infected
sheep with neurological signs in California. In contrast to horses, a large
amount of viral antigens accumulated in the CNS of sheep in this study. As a
result, both PCR and IHC were useful testing modalities in these sheep. Viral
antigens in avian species are widespread and the contributor provides excellent
quality images of strong positive cytoplasmic immunoreactivity for WNV antigen
within intestinal epithelial cells and crypts and pancreatic acinar cells.
After injection
of the virus by the mosquito vector, the virus is deposited in the
extracellular matrix where it can propagate in keratinocytes and infect
Langerhans dendritic cells and tissue macrophages. The virus then spreads
hematogenously via the leukocyte tracking system. Viral envelope proteins E2
and E1 are responsible for organ attachment and endocytosis respectively.
Although not completely understood, entry of the virus into the CNS likely
involves a combination of breakdown of the blood-brain barrier by
proinflammatory cytokines and retrograde axonal transport from the peripheral
nervous system.
References:
1. Austin RJ,
Whiting TL, Anderson RA, Drebot MA. An outbreak of West Nile virus-associated
disease in domestic geese (
Anser anser domesticus) upon initial
introduction to a geographic region, with evidence of bird to bird
transmission.
Can Vet J. 2004; 45(2):11-23.
2. Banet-Noach C,
Simanov L, Malkinson M. Direct (non-vector) transmission of West Nile virus in
geese.
Avian Pathol. 2003; 32(5):489-94.
3. Cantile C,
Youssef S. Nervous system. In: Maxie MG, ed.
Jubb, Kennedy, and Palmers
Pathology of Domestic Animals. Vol 1. 6th ed. St. Louis, MO: Elsevier;
2016:374-375.
4. Gamino V, Höfle
U. Pathology and tissue tropism of natural West Nile virus infection in birds:
a review.
Vet Res. 2013; 44:39.
5. Miller AD,
Zachary JF. Nervous System. In: McGavin MD, Zachary JF, eds.
Pathologic
Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier Mosby;
2017:876-877.
6. Nemeth NM, Brown
JD, Stallknecht DE, Howerth EW, Newman SH, Swayne DE. Experimental infection of
bar-headed geese (
Anser indicus) and ruddy shelducks (
Tadorna
ferruginea) with a clade 2.3.2 H5N1 highly pathogenic avian influenza
virus.
Vet Pathol. 2013; 50(6):961-70.
7. Pérez-Ramírez E,
Llorente F, Jiménez-Clavero MÁ. Experimental infections of wild birds with West
Nile virus.
Viruses. 2014; 6(2):752-81.
8. Swayne DE, Beck
JR, Smith CS, Shieh WJ, Zaki SR. Fatal encephalitis and myocarditis in young
domestic geese (
Anser anser domesticus) caused by West Nile virus.
Emerg
Infect Dis. 2001; 7(4):751-3.
9. Zachary JF.
Mechanisms of microbial infection. In: Zachary JF ed.
Pathologic Basis of
Veterinary Disease. 6th ed. St. Louis, MO: Mosby Elsevier; 2017:223.
10. Zhou JY, Shen
HG, Chen HX, Tong GZ, Liao M, Yang HC, Liu JX. Characterization of a highly
pathogenic H5N1 influenza virus derived from bar-headed geese in China.
J
Gen Virol. 2006; 87:1823-33.
11.
12.