WSC 2023-24 Conference 11, Case 3
Signalment:
Fetus aborted at approximately 120 days of gestation, ovine, (Ovis aries)
History:
In July 2015, two of approximately 200 pastured sheep in a family farm in Colonia, Uruguay, aborted three fetuses at approximately 4 months of gestation.
Gross Pathology:
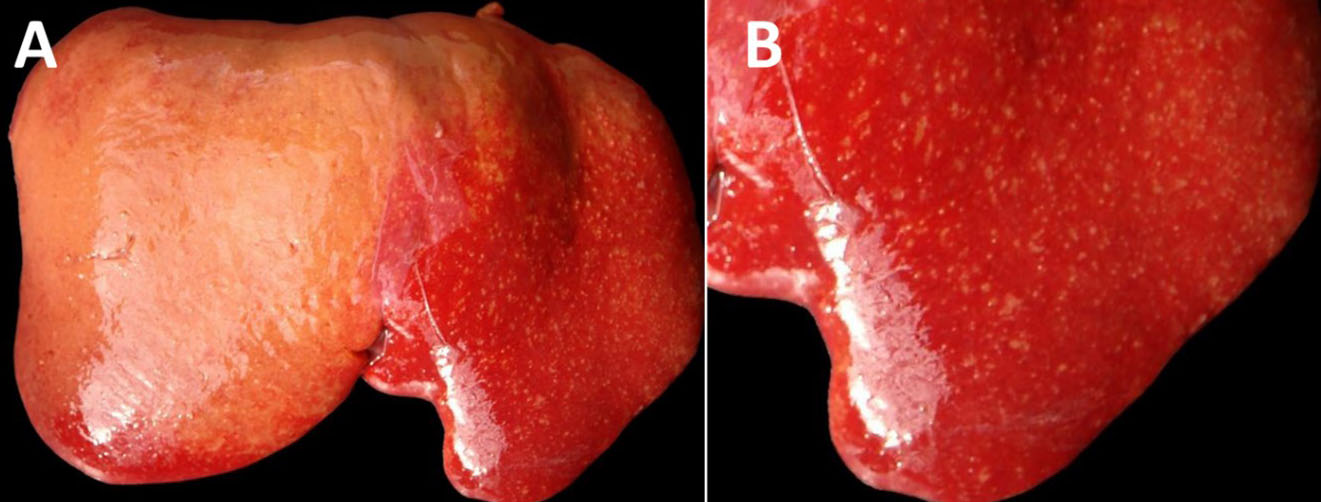
The fetus was a female, with a crown-to-rump length of 32 cm (estimated gestational age: 120 days) with a complete wool/hair coat, in a moderate state of post-mortem decomposition (moderate autolysis). The fetus was fully formed, with a complete wool/hair coat and no external abnormalities. The liver was moderately enlarged, with rounded edges, and there were numerous, discrete, white to yellowish, pinpoint to ≤2 mm foci throughout the hepatic parenchyma with a multifocal widespread (disseminated) distribution (necrotizing hepatitis). There were fibrin strands in the abdominal and pericardial cavities (moderate fibrinous peritonitis and pericarditis). No other gross lesions were noted. The lungs were unventilated/unexpanded, and there were no colostrum/milk curds in the lumen of the abomasum.
Laboratory Results:
IHC using a monoclonal primary antibody against Francisella tularensis lipopolysaccharide (LPS) revealed abundant and strong immunoreactivity colocalizing extracellularly with the hepatic lesions and intracellularly in infiltrating inflammatory cells.
Microscopic Description:
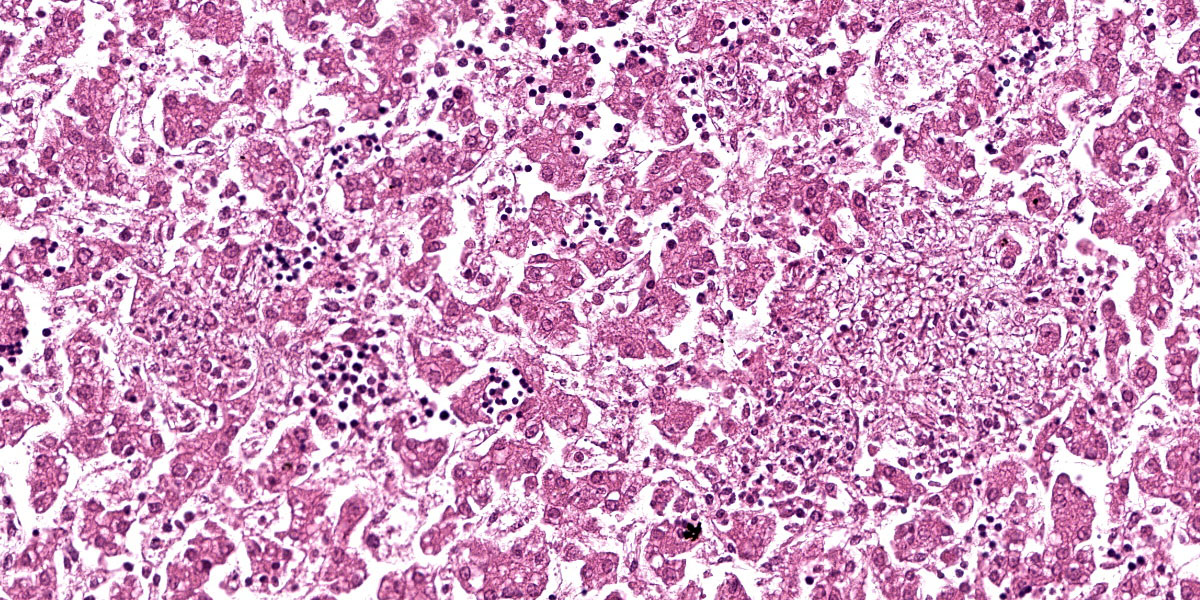
Liver: Randomly throughout the parenchyma, there are multiple foci of necrosis and inflammation. These foci are characterized by disruption and effacement of the hepatic cord architecture and replacement by necrotic cell debris; the hepatocytes have angular cell borders, hypereosinophilic cytoplasm, and either nuclear pyknosis or karyorrhexis (necrosis). Within these foci, there is an accumulation of eosinophilic fibrillar material (fibrin), and inflammatory cell infiltrates, mostly neutrophils and macrophages. There is an increased number of neutrophils in the sinusoids (circulating neutrophilia).
Contributor’s Morphologic Diagnosis:
Liver: Hepatitis, necrotizing and fibrinosuppurative, multifocal widespread, random, severe.
Contributor’s Comment:
The hepatic lesions in this fetus were suggestive of an infectious etiology. In addition, the microscopic examination of other fetal tissues revealed multifocal neutrophilic myocarditis, multifocal neutrophilic bronchiolitis and alveolitis, and fibrinous splenic capsulitis (peritonitis). Altogether, these lesions were suggestive of a bacterial cause.
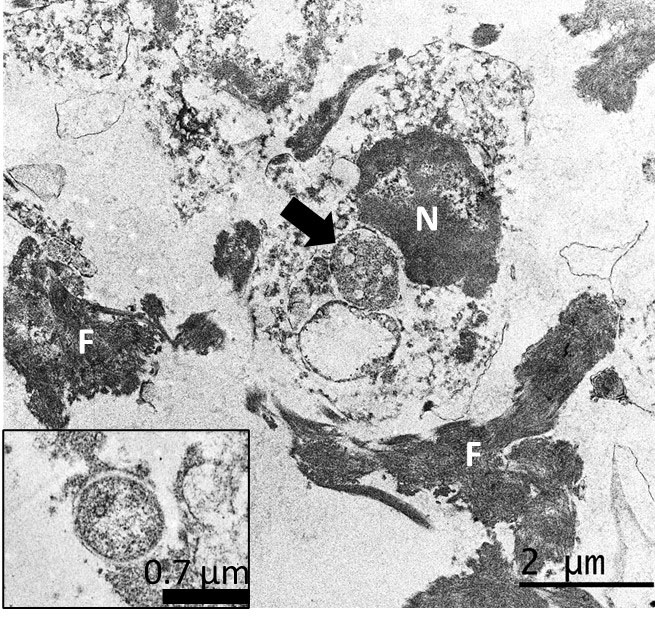
Ancillary testing to assess for possible abortifacients of sheep, in this case, included immunohistochemistry (IHC) for Chlamydia spp., Listeria monocytogenes, Coxiella burnetii, Salmonella enterica, and Toxoplasma gondii, all of which yielded negative results. Additionally, no curved bacilli (i.e., Campylobacter), spirochetes (i.e., Leptospira, Flexispira) or fungi were identified in sections of liver stained with Steiner silver stain and Gomori´s methenamine silver stain, and no intralesional bacteria were visualized with tissue Gram stain. However, IHC using a monoclonal primary antibody against Francisella tularensis lipopolysaccharide (LPS) revealed abundant and strong immunoreactivity colocalizing extracellularly with the hepatic lesions and intracellularly in infiltrating inflammatory cells. Finally, transmission electron microscopy on formalin-fixed paraffin-embedded liver revealed intraphagocytic (intrahistiocytic) and extracellular ~0.7–1.7 μm coccobacilli (expected size for Francisella spp.) in the foci of necrotizing hepatitis. Details of the diagnostic investigation conducted in this case were recently published.5
The Francisellaceae family comprises gram-negative coccobacilli and four genera are currently recognized: Francisella, Allofrancisella, Pseudofrancisella, and Cysteiniphilum, of which only Francisella is currently considered of clinical relevance. Francisella tularensis is the most studied species because it causes tularemia, a highly transmissible, potentially life-threatening, zoonotic disease, also considered a potential bioterrorism agent.8 Tularemia occurs over almost the entire Northern Hemisphere but is rarely reported in the Southern Hemisphere, where the only published cases have occurred in Australia.4
Although F. tularensis has a broad host range, sheep have been considered the only livestock species affected by epizootics of tularemia and have been implicated in disease transmission to sheep industry workers.7,10 The abortifacient effects of F. tularensis in sheep have been described in the United States, and tularemia has been regarded as an overlooked syndrome in sheep.10
From a pathologic viewpoint, necrotic foci in the liver, spleen, or lungs in late term aborted ovine fetuses are characteristic of tularemia and should raise suspicion, although gross lesions can be absent even in cases with typical histologic inflammatory and necrotizing lesions.10 Contrary to most bacterial abortifacients of sheep, F. tularensis is not visible upon histopathologic examination of tissues stained with hematoxylin and eosin, Steiner silver stain or Gram stain, even in tissues that have a high bacterial burden demonstrated by IHC.3,10 The diagnostic investigation of any case of ovine abortion with fetal lesions indicating a bacterial etiology should include ancillary testing to identify F. tularensis and rule out other abortigenic pathogens.3
The etiologic diagnosis in our case was reached by the immunohistochemical demonstration of abundant intralesional antigen by a specific monoclonal antibody raised against F. tularensis LPS. IHC has proven useful for identifying F. tularensis in diagnostic settings.6,10 F. tularensis LPS is a main specific antigen and virulence factor and differs from the LPS of other gram-negative bacteria.9 According to the manufacturer, the primary antibody used in this case for the IHC does not cross-react with Francisella novicida, Yersinia pestis, Y. pseudotuberculosis, Y. enterocolitica, Vibrio cholerae, Escherichia coli, Salmonella enterica serovar Typhimurium, Brucella abortus, B. suis, B. ovis, B. melitensis, or B. neotomae. We tested the IHC in cases of ovine abortion caused by Campylobacter jejuni and C. fetus but observed no cross-reactivity with these pathogens. Although cross-reaction with other members of Francisellaceae cannot be completely ruled out, F. tularensis is currently the only species of this family recognized as an ovine abortifacient. Definite species and sub-species identification requires bacterial isolation and DNA analysis, which we were unable to perform in this case. The ultrastructural demonstration of intracellular gram-negative coccobacilli of the expected size in phagocytic and inflammatory cells in tissues with lesions, as in our case, aids in the diagnosis, but is by no means confirmatory.
The lack of historical reports of tularemia outside endemic areas of North America and Eurasia has been puzzling.4 Recently, tularemia emerged in Australia and reemerged in the Northern Hemisphere.8,15 South America has been considered free of tularemia, which seems to be based solely on the lack of disease reporting.15 However, tularemia might have been undiagnosed because of limitations in disease surveillance systems in the region. No clinical disease caused by Francisella spp. in mammals in the Americas south of Mexico has been described. This case raised concerns about the possible occurrence of tularemia in South America.5
The source of infection in this sheep remains unknown; however, F.tularensis has a broad range of animal reservoirs, including arthropods, rodents, lagomorphs, and marsupials.4,12 Brown hares (Lepus europaeus), a species that plays a primary role in the ecology of tularemia in Europe, have been introduced to Uruguay and are frequently seen around the affected farm.6 In addition, F. tularensis can be transmitted by ticks, including Amblyomma spp., Haemophysalis spp., and Ixodes spp. ticks, which are endemic in Uruguay. Of note, a gamma-proteobacterium related to Francisella-like organisms, but different from F. tularensis, was identified in Uruguay in Amblyomma triste ticks, the most prevalent tick species reported in human tick bites in the country.14
Contributing Institution:
Plataforma de Investigación en Salud Animal,
Instituto Nacional de Investigación
Agropecuaria (INIA),
La Estanzuela, Uruguay
http://www.inia.uy
JPC Diagnosis:
- Liver: Hepatitis, necrotizing, multifocal and random, moderate.
- Liver: Extramedullary hematopoiesis, diffuse, moderate.
JPC Comment:
Francisella tularensis is poorly staining gram-negative coccobacillus that is non-motile, aerobic, and is a facultative intracellular pathogen. There are three subspecies that vary in their geographic distribution and virulence. F. tularensis subsp. tularensis accounts for the majority of clinical infections in domestic animals, is highly pathogenic, and is found in North America and Europe.11 Two less virulent strains, F. tularensis subsp. holartica and F. tularensis subsp. mediasiatica occur in Europe/North America and Asia, respectively. Interestingly, subsp. holartica is frequently linked to aquatic mammals such as beavers and muskrats, and its reservoir is considered to be a protozoal organism rather than a mammal.11
In vivo, the organism enters host macrophages, where it arrests the maturation and acidification of the phagolysosome.11 The organism then escapes the phagosome and replicates freely in the cytoplasm, where it can trigger either caspase 3-mediated apoptosis or an inflammatory form of host cell death called pyroptosis, resulting in release of bacteria that can initiate another round of host cell infection.2,11 The ability of F. tularensis to survive the phagosome is dependent on the gene products of a 30kb stretch of bacterial DNA, known as the Francisella Pathogenicity Island (FPI), that encodes 18 genes, 14 of which are required for growth within and escape from macrophages.2 Among the virulence factors encoded on the FPI is a recently discovered Type VI secretion system which is required for macrophage escape and intracytoplasmic replication.1 F. tularensis organisms that are deficient in key pieces of this secretion nanomachine are rendered essentially avirulent in mouse models.1
If all is working well, however, F. tularensis can cause fulminating, fatal disease in wildlife species, domestic animals, and humans. In most species, the disease is characterized by fever, depression, inappetence, and manifestations of septicemia. Outbreaks have been reported in sheep, horses, and young pigs, while adult pigs, cattle, and dogs appear to be relatively resistant to infection.11
Cats are the domestic animal most associated with disease, with clinical forms (typhoidal, respiratory, ulceroglandular, and oropharyngeal) mirroring those described in humans.11 Grossly, tularemia in cats is characterized by sizeable (2mm diameter or more), miliary white foci in the liver, spleen, and lymph nodes.13 Histologically, lesions are identical to those presented here, with focal areas of severe necrosis with bacteria that are difficult to visualize on H&E section.13
Conference discussion focused largely on the typical presentations and pathogenesis of tularemia in various species. From a diagnostic standpoint, it is important to remember that, unlike most infectious differentials for hepatic necrosis, F. tularensis is, rather unhelpfully, not visible on H&E, Steiner, or Gram stains, even in tissues with high bacterial burdens demonstrated with immunohistochemical staining. Dr. Travis also discussed documented human cases of tularemia contracted from “face snuggling” domestic dogs (“if we die, we die”) and from aerosolized infective material liberated from wildlife carcasses during yard work.
Despite the absence of demonstrable organisms, this case provides an excellent example of the hepatic manifestation of gram-negative sepsis. Participants noted multifocal areas of extramedullary hematopoiesis within sinusoids, which was felt substantial enough to include in the morphologic diagnoses.
References:
- Brodmann M, Dreier RF, Broz P, Basler M. Francisella requires dynamic type VI secretion system and ClpB to deliver effectors for phagosomal escape. Nat Commun. 2017;8:15853.
- Clemens DL, Lee BY, Horwitz MA. The Francisella type VI secretion system. Front Cell Infect Microbiol. 2018;8: 00121.
- Dorsch MA, Cantón GJ, Driemeier D, Anderson ML, Moeller RB, Giannitti F. Bacterial, protozoal and viral abortions in sheep and goats in South America: a review. Small Rumin Res. 2021;205 :106547.
- Eden JS, Rose K, Ng J, et al. Francisella tularensis ssp.holarctica in Ringtail Possums, Australia. Emerg Infect Dis. 2017; 23:1198-1201.
- Giannitti F, Dorsch MA, Schild CO, et al. Pathologic and immunohistochemical evidence of possible Francisellaceae among aborted ovine fetuses, Uruguay. Emerg Infect Dis. 2023;29:141-144.
- Gyuranecz M, Szeredi L, Makrai L, et al. Tularemia of European Brown Hare (Lepus europaeus): a pathological, histopathological,and immunohistochemical study. Vet Pathol. 2010;47:958-63.
- Jellison WL, Kohl GM. Tularemia in sheep and in sheep industry workers in western United States. Public Health Monogr. 1955;28:1-19.
- Maurin M. Francisella tularensis as a potential agent of bioterrorism? Expert Rev Anti Infect Ther. 2015;13:141-144.
- Okan NA, Kasper DL. The atypical lipopolysaccharide of Francisella. Carbohydr Res. 2013; 378:79-83.
- O'Toole D, Williams ES, Woods LW, et al. Tularemia in range sheep: an overlooked syndrome? J Vet Diag Invest. 2008;20:508-513.
- Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning S, Hartigan PJ. Veterinary Microbiology and Microbial Disease. 2nd ed. Blackwell;2011.
- Sjöstedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007; 1105:1-29.
- Valli VEO, Kiupel M, Bienzle D. Hematopoietic System. In: Maxie GM, ed. Vol. 3, Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Elsevier, Inc;2016:184-186.
- Venzal JM, Estrada-Peña A, Portillo A, et al. Detection of Alpha and Gamma-Proteobacteria in Amblyomma triste (Acari: Ixodidae) from Uruguay. Exp Appl Acarol. 2008;44:49-56.
- Yeni DK, Büyük F, Ashraf A, Shah MSUD. Tularemia: a re-emerging tick-borne infectious diseas. Folia Microbiol (Praha). 2021;66:1-14.