Signalment:
Gross Description:
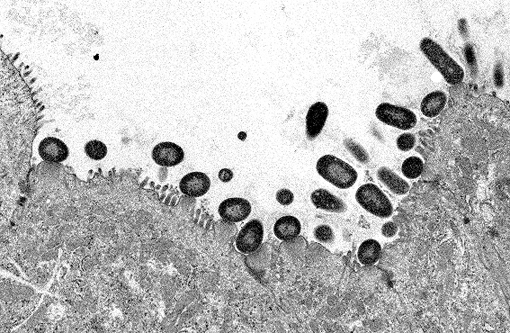
Electron microscopy: Intestine: The image consists of portions of four enterocytes, identified by the presence of numerous normal and swollen microvilli. Some of the microvillar border is replaced by randomly arranged, oval to elongate, approximately 0.8 to 1.0 μm diameter, encapsulated bacilli. The bacilli are located outside the cell membrane on the apical surface of enterocyte where they create an indentation (cup and/or pedestal) where they abut the cell surface. At the attachment site, the apical portion of affected enterocytes is thickened by a homogeneous band of lightly electron dense material that disrupts the terminal web. In the swollen microvilli, the actin cystoskeleton is not recognizable. Both nucleated cells present in the image exhibit mild hydropic changes in the apical cytoplasm, indicative of degeneration.
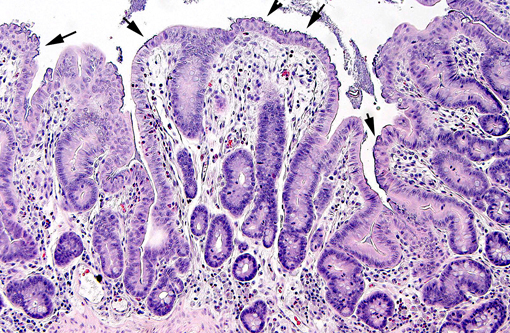
Histopathologic Description:
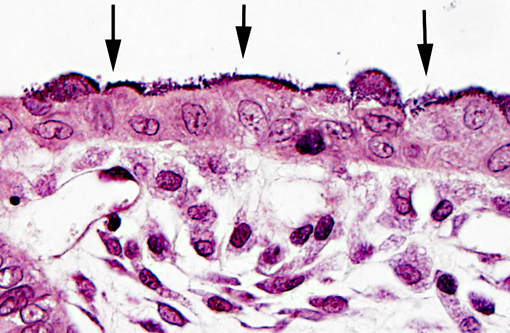
Bacteria attached to the apical surface of enterocytes are gram-negative bacilli.
Other findings include renal tubular necrosis and regeneration, hepatic and myocardial necrosis with mineralization, focal heterophilic subpleural pneumonia, and tracheitis.
Morphologic Diagnosis:
Condition:
Contributor Comment:
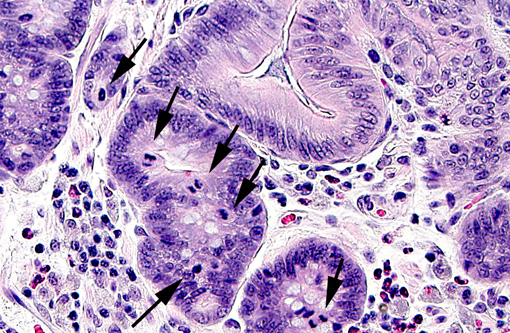
In weaned rabbits, bacterial attachment occurs in the ileum, cecum and colon. Weanling rabbits are typically affected at 4-6 weeks of age; diarrhea occurs approximately six days after infection and the gross findings include watery intestinal contents, serosal ecchymosis, edema of the intestinal walls and mesenteric lymphadenomegaly. Microscopically, bacteria adhere to enterocytes and have a typical bacillary appearance. Colonized cells appear degenerate and many are hyperchromatic, rounded-up and/or pyknotic, with frequent mucosal erosions and detachment. At low magnification, this confers a cobblestone appearance to the mucosal surface. The inflammatory component of the lesion is variable, ranging from mild edema to focal polymorphonuclear infiltrates infiltrating the lamina propria.(9,11) Serotypes frequently found in weaned rabbits are O15:H-, O26:H11, O103:H2 and O109:H2.(8,9)
Initial attachment of the bacteria is non-intimate and restricted to the follicle-associated epithelium of Peyers patches in the ileum, hence the differences of strain causing disease in suckling and weanlings rabbits. It has been proposed that the resistance of suckling rabbits to weanling-associated strains is due to the fact that Peyers patches do not develop before two weeks of age.(3)
Primary non-intimate adhesion is critical for the virulence of rabbit strains of EPEC, and is accomplished by a variety of adhesins, often targeted at the follicle associated epithelium of the ileal Peyers patches, similar to the process observed in humans.(10) Rabbit AEEC rarely produce enterotoxin or Verotoxin and in contrast to other host species, the presence of eae gene in the rabbit is closely associated with diarrheal disease.(2)
The attaching-effacing lesion is due to an interesting mechanism, which is well studied in vitro using enteropathogenic E. coli (EPEC) O127:H6. This mechanism consists of four stages, responsible for the pathologic and ultrastructural findings (reviewed by Wales et al, 2005).(11) Briefly, the first stage is initial non-intimate attachment. EPEC secreted proteins (Esp) Esp-A, -B and -D are exported via a type III secretion system into the host eukaryotic cell. Esp-B and -D, in addition, may create a pore forming structure in the cell membrane, and these elements form a channel for the introduction of bacterial macromolecules in the host cytoplasm. During this stage, there is translocation of the translocated-intimin-receptor protein (Tir) into the host cell. During the second stage, there is signal transduction leading to cytoskeletal reorganization and microvillus effacement. At the site of intimate attachment, there is considerable cytoskeletal reorganization, with depolarization of actin, formation of F-actin and accumulation of α-actinin, myosin light chain, talin and ezrin; all of these changes are associated with effacement of microvilli and disruption of the intestinal barrier function of enterocytes. During this second stage, Tir is inserted in host cell membrane. The third stage is known as intimate attachment. In the third stage, Tir focuses filamentous actin, forming a pedestal. Intimin, encoded by the enterocyte attaching and effacing gene (eae), is a surface-exposed outer membrane protein. Intimin binds Tir, and this binding is indispensable for the development of AE lesions. The fourth stage is invasion. Intracytoplasmic bacteria have been observed in vacuoles and free in the cytoplasm of the host cell, and rarely in the lamina propria. In the rabbit AEEC is not considered to be enteroinvasive.(9)
In conclusion, even in absence of bacteriologic culture, the diagnosis of this case was possible based on the histopathological and electron microscopy findings. Culture and serotyping of the bacteria, plus histopathology, are the preferred methods of diagnosis. No other cases were identified in the colony after this event.
JPC Diagnosis:
Conference Comment:
Esherichia coli is a gram-negative, non-spore-forming bacillus. Non-virulent strains are often part of the normal intestinal flora, while virulent strains are capable of causing multiple disease syndromes in humans and animals via assorted combinations of virulence factors.(1,4) Specific serotypes of E. coli (e.g., O157:H7) are named based on the presence of the following antigens:(4)
- O-antigen (somatic): located on the lipopolysaccharide molecule
- K-antigen (capsular): outermost structural component composed of carbohydrates
- H-antigen (flagellar): composed of flagellin protein
- F-antigen (fimbrae or pili): adhesins which project from the bacterial cell wall
Enteric disease is a common manifestation of colibacillosis. The pathology of enteropathogenic, or attaching and effacing (AEEC) E. coli is described in detail by the contributor. In addition to rabbits, this form of colibacillosis can cause diarrhea in pigs, dogs and humans. Some strains of enterohemorrhagic E. coli (EHEC), also known as Shiga toxin-producing or verotoxin-producing E. coli, are attaching and effacing as well. This subset produces cytotoxins that are structurally similar to the Shiga toxins generated by Shigella dysenteriae, which bind the host cell glycolipid receptor globotriaosylceramide (Gb3), causing inhibition of protein synthesis with subsequent necrosis. Shiga toxins primarily affect intestinal epithelium and vascular endothelium, due to the presence of Gb3 receptors in these tissues. Enterohemorrhagic strains of E. coli, such asserotype O157:H7, are most notorious as a cause of human foodborne illness; however, they are also associated with erosive fibrinohemorrhagic enterocolitis in calves under four weeks old. In dogs, EHEC has occasionally been linked with dysentery and, in greyhounds, a hemolytic uremic syndrome with cutaneous edema and ulceration known as cutaneous and renal glomerular vasculopathy. Furthermore, edema disease in weaned pigs is caused by enteric colonization with Shiga toxin-producing E. coli, mediated by F18ab fimbriae. This syndrome is characterized by central nervous system signs or sudden death due to vasculitis and edema. It is often associated with outbreaks of post-weaning E. coli enteritis (EHEC) and only occasionally produces diarrhea. Typically, edema disease results in a classical enterotoxemia without significant gross or microscopic lesions.(1)
Enterotoxigenic E. coli (ETEC) is a common cause of neonatal diarrhea in calves and piglets less than four days old. Among its most important virulence factors is its ability to colonize the intestine and produce enterotoxin. Fimbriae (also known as pili), composed of pilin, project from the bacterial cell wall, and attach to enterocyte surface receptors. Fimbrial adhesins are antigenically distinct and somewhat species-specific; immunohistochemical stains can thus be helpful in reaching a definitive diagnosis. Enterotoxins produced by ETEC include heat-labile toxin and heat-stable toxin. Heat-labile toxin is composed of two subunits, known as LTI and LTII. LTI resembles cholera toxin, while LTII utilizes an adenylate cyclase pathway (i.e., cAMP) to initiate irreversible intestinal secretion of electrolytes and water, leading to secretory diarrhea. The heat-stable toxin STa causes inhibition of Na/Cl co-transport and water absorption via an increase in cyclic guanosine monophosphate (cGMP), while STb (primarily associated with ETEC in pigs) promotes secretion by stimulating production of prostaglandin E2 and 5-hydroxytrypamine. Although ETEC results in severe osmotic diarrhea, dehydration, and potentially death, this syndrome serves as another example of a classical enterotoxemia with minimal gross and microscopic lesions.(1)
Some strains of E. coli are able to invade intestinal enterocytes, eventually disseminating throughout the body and resulting in septicemic colibacillosis. These enteroinvasive strains are best described in humans; however, invasive strains of E. coli also affect fowl, resulting in myriad clinical syndromes including septicemia and enteric disease.(4) Other strains of E. coli induce systemic infection by avoiding host defense mechanisms, especially in young, compromised animals such as neonates subjected to failure of passive transfer of immunity, or those with concurrent, debilitating disease. Several important virulence factors of potentially septicemic E. coli strains, such as the capsule, outer membrane proteins, toxins, or fimbriae, are encoded on bacterial colicin V plasmids. When these bacteria die they often release endotoxin from lipopolysaccharide on the outer membrane, leading to the clinical signs and gross and microscopic lesions associated with endotoxic shock.(1)
References:
2. Blanco JE, Blanco M, Blanco J, Mora A, Balaguer L, Mourino M, Juarez A, et al. O serogroups, biotypes and eae genes in Escherichia coli strains isolated from diarrheic and healthy rabbits. J Clin Microbiol. 1996;34:3101-3107.
3. Heczko U, Abe A, Finlay BB. In vivo interaction of rabbit enteropathogenic Escherichia coli O103 with its host: electron microscopic and histopathologic study. Microbes Infect. 2000;2:5-16.
4. Hirsh DC. Family Enterobacteriaceae. In: Hirsh DC, Zee YC, eds. Veterinary Microbiology. Malden, MA: Blackwell Science; 1999:69-74.
5. Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340-1351.
6. Peeters JE, Charlier GJ, Raeymaekers R. Scanning and transmission electron microscopy of attaching effacing Escherichia coli in weanling rabbits. Vet Pathol. 1985;22:54-59.
7. Peeters JE, Geeroms R, Ǿrskov F. Biotype, serotype, and pathogenicity of attaching and effacing enteropathogenic Escherichia coli strains isolated from diarrheic commercial rabbits. Infect Immun. 1988;56:1442-1448.
8. Peeters JE, Pohl P, Okerman L, Devriese LA. Pathogenic properties of Escherichia coli strains isolated from diarrheic commercial rabbits. J Clin Microbiol. 1984;20:34-39.
9. Percy DH, Barthold SW. Pathology of laboratory rodents and rabbits. 3rd ed. Ames, IA: Blackwell Publishing; 2007:273-274.Â
10. Phillips AD, Frankel G. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organs in culture. J Infect Dis. 2000;181:1496-1500.
11. Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals. J Comp Path. 2005;132:1-26.