Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 13
January 3rd, 2018
CASE IV: NE 17-399 (JPC 4101750).
Signalment: 8.5 year-old, female, African black-footed (jackass) penguin (Spheniscus demersus).
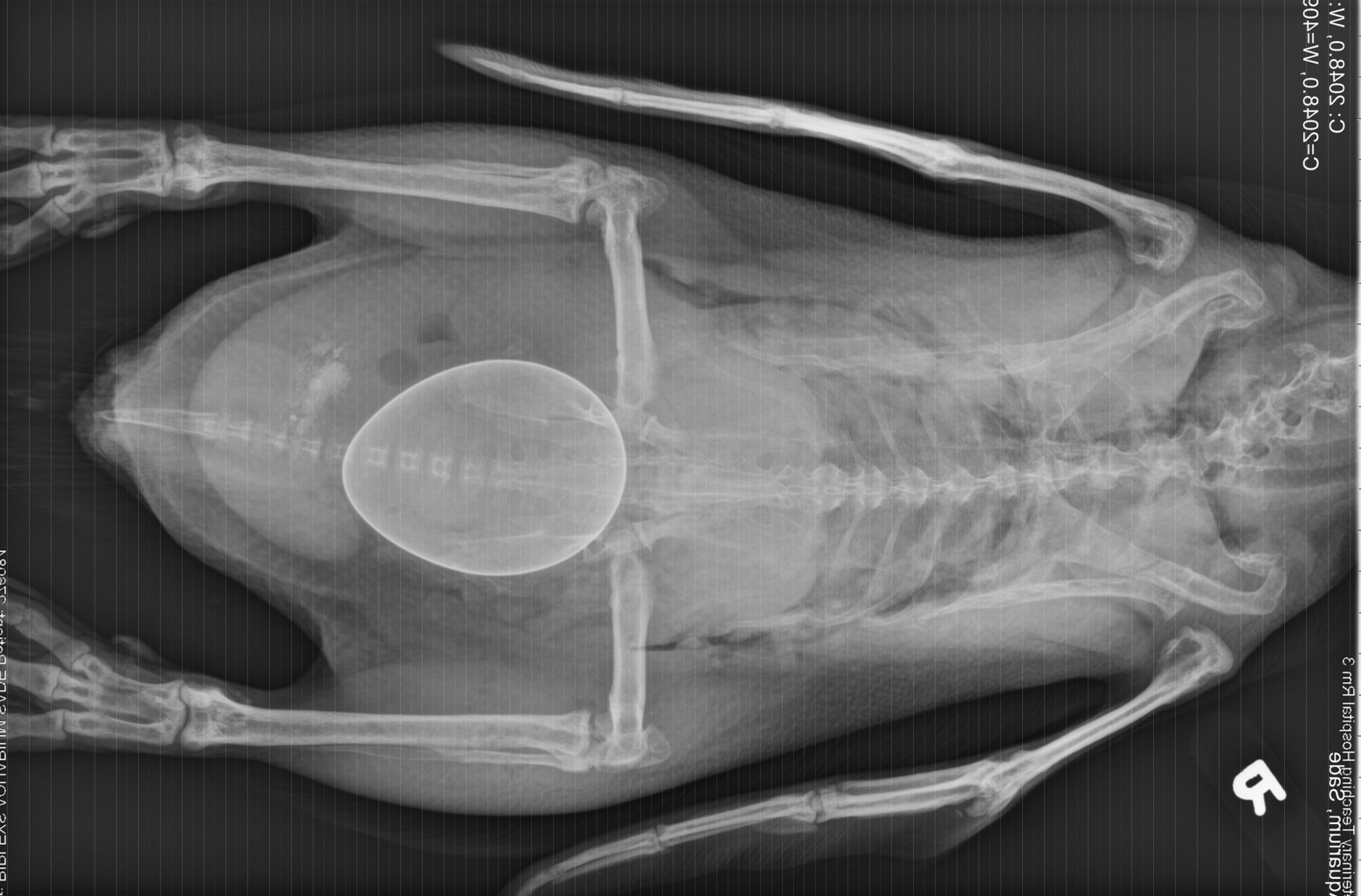
History: This penguin was presented for acute inability to walk 2 days after laying an egg. Radiographs showed a large shelled egg in the coelom and diffuse osteopenia compared to an age-matched, control penguin. She was treated with calcium, butorphanol, meloxicam, enrofloxacin, and subcutaneous fluids, but died several hours later.
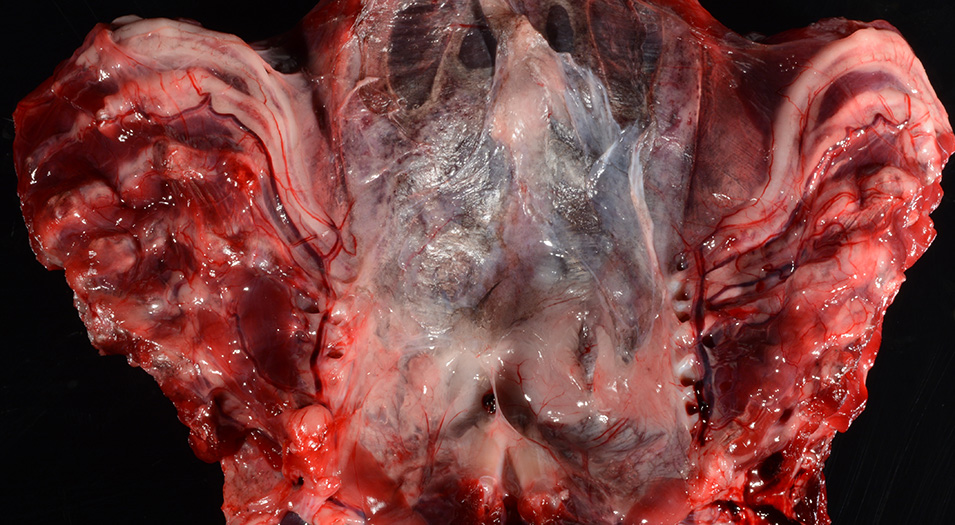
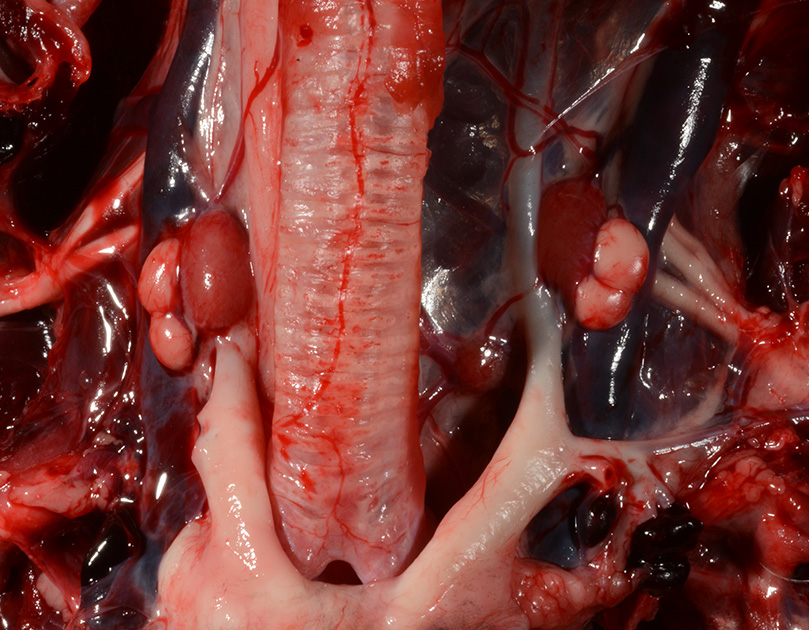
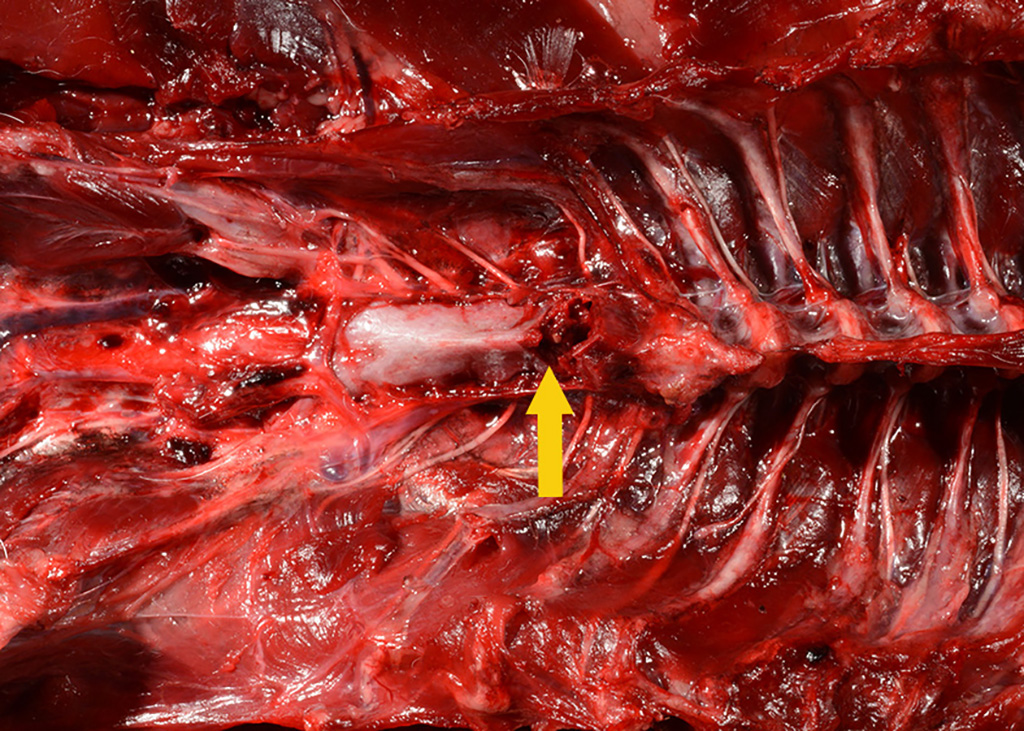
Gross Pathology: The body was in good nutritional condition (body condition score 3/5). The ribs were irregular, soft and serpentine, and cut easily with a scalpel blade. Both the left and right parathyroid glands were enlarged; the left measured 9x4x1.5mm and the right measured 10x4x1.5mm. There was an 89.7g egg in the oviduct near the cloaca. The eggshell was pale blue-green and hard. There was a complete fracture of the spine at the synsacral-thoracic junction with associated hemorrhage.
Laboratory results:
None provided.
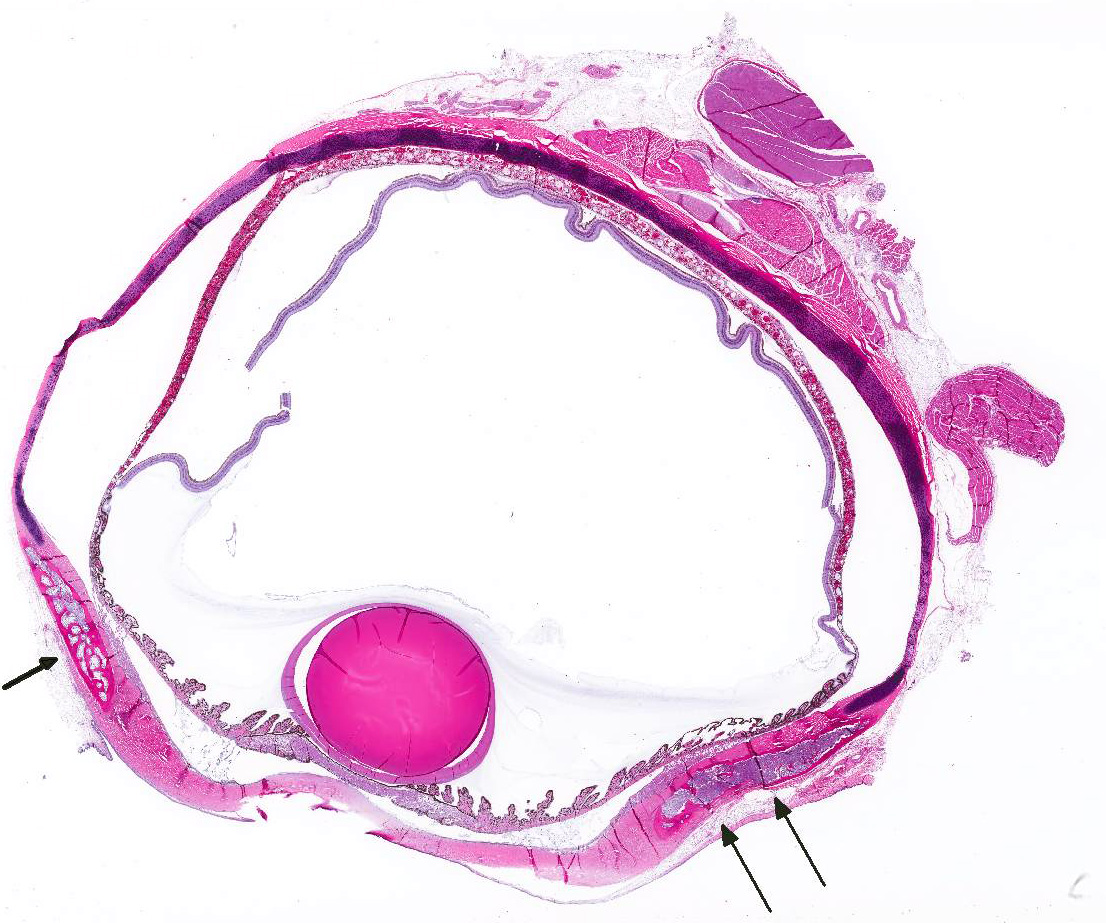
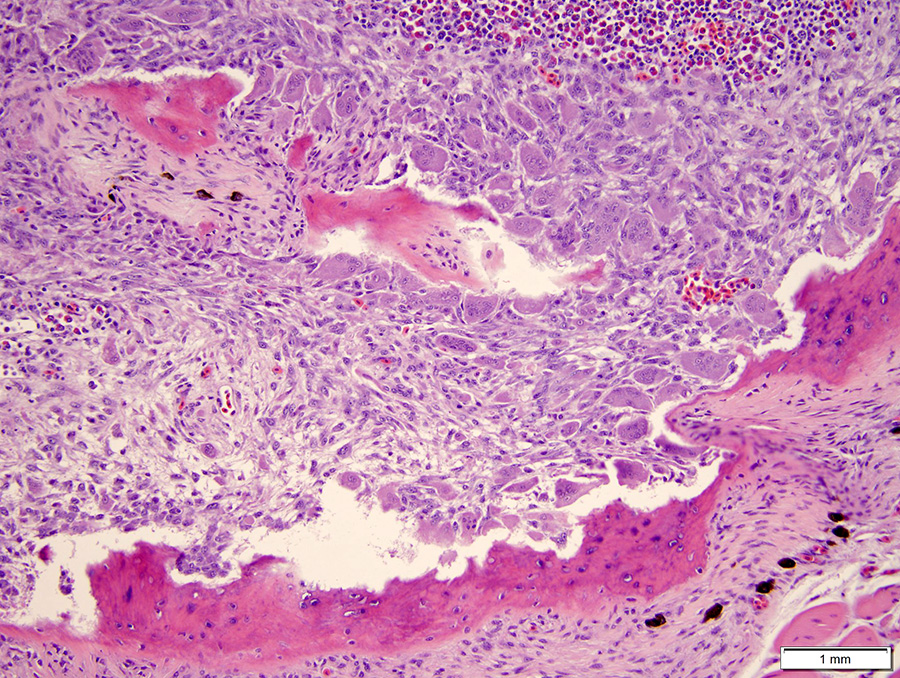
Microscopic Description: The hematopoietic and adipose tissue of the marrow of the scleral ossicles is variably replaced by fibroblasts and osteoclasts. The numerous osteoclasts and their Howship’s lacunae are forming a scalloped endosteal margin and thin cortex.
Contributor’s Morphologic Diagnoses:
Scleral ossicles: Marked osteolysis with fibroplasia (fibrous osteodystrophy).
Contributor’s Comment: This was a case of nutritional secondary hyperparathyroidism with widespread fibrous osteodystrophy and vertebral fracture. The lesions in the scleral ossicles were also present in the femur, ribs, and vertebrae (all other bones examined).
This penguin was part of an indoor colony at an aquarium. The diet consisted of capelin and a daily avian multivitamin. At one time this bird was supplemented with calcium carbonate during times of egg production, but due to her habit of only laying a single egg per season, the supplementation was discontinued. It is common for penguins to lay two eggs per clutch. After the death of this bird, serum from other birds in the collection was tested for calcium and vitamin D3 levels; both were low and the birds are now supplemented.
Nutritional hyperparathyroidism and fibrous osteodystrophy has been reported in juvenile penguins.1 It also occurs in laying hens, where the physiology of parathyroid hormone (PTH) is like that in mammals. PTH responds to hypocalcemia by stimulating osteoclasts (directly and indirectly via osteoblasts) to remove bone.3 The response is especially rapid in birds, where in response to PTH, osteoclasts increase their cell spread area by 40% within 2-4 minutes.3 Osteoclasts also increase their ruffled border and acid production in response to PTH.3
JPC Diagnosis: Eye, scleral ossicles: Osteolysis, diffuse, severe with fibroplasia (fibrous osteodystrophy), African black-footed (jackass) penguin (Spheniscus demersus).
Conference Comment: Fibrous osteodystrophy is a metabolic bone disease characterized by bone resorption with proliferation of adjacent fibrous tissue and poorly mineralized immature bone. This condition is due to chronically elevated plasma parathyroid hormone (PTH) or hyperparathyroidism and occurs in horses, pigs, dogs, cats, ferrets, goats, reptiles, and non-human primates (sheep and goats are relatively unaffected). PTH can be elevated due to any one of the following: functional parathyroid gland adenoma (primary hyperparathyroidism), hypercalcemia of malignancy (production of parathyroid hormone-related peptide (PTHrP)), reduced renal clearance of phosphate and thus low calcium due to their inverse relationship (renal secondary hyperparathyroidism), dietary deficiency of calcium, excess of phosphorus, or in association with vitamin D deficiency (nutritional secondary hyperparathyroidism).2
Primary hyperparathyroidism is characterized by autonomous secretion of PTH resulting in persistent hypercalcemia and hypophosphatemia (due to increased urinary secretion of phosphate). This clinical pathology finding distinguishes primary from secondary hyperparathyroidism. In all cases of secondary hyperparathyroidism, plasma total calcium concentrations are normal or slightly decreased. Animals with primary hyperparathyroidism often suffer from polyuria and polydipsia, generalized muscle weakness, and widespread mineralization of soft tissues including nephrocalcinosis, which may result in the death of the animal even before skeletal changes are evident. Hereditary and familial primary hyperparathyroidism has been reported in German shepherd puppies and Keeshond dogs, respectively. Hypercalcemia of malignancy due to elevated PTHrP is most common in lymphoma and apocrine gland adenocarcinoma of the anal sac.2
Renal secondary hyperparathyroidism develops when impaired glomerular filtration leads to decreased excretion of phosphate. Hyperphosphatemia and is more common in dogs and cats. Subsequently, hypocalcemia develops (due to complexing of calcium with phosphate) which stimulates the release of PTH. Additionally, the hypocalcemia is exacerbated by released of FGF23 from osteocytes (triggered by hyperphosphatemia) with increases renal excretion of phosphate and suppresses 1α-hydroxylase which leads to decreased production and breakdown of 1,25(OH)2D3. Adult dogs with renal failure are most commonly affected with renal secondary hyperparathyroidism, but, as mentioned above, skeletal lesions are usually not as clinically important as the manifestations of uremia.2
Finally, nutritional secondary hyperparathyroidism is most commonly caused by diets with low calcium and a high concentration of phosphorus. In most species, this affects primarily young, rapidly growing animals except in horses that are extremely sensitive to the effects of high phosphorus. In horses, nutritional secondary hyperparathyroidism, colloquially known as “bran disease” usually occurs after several months of a maintenance diet high in grain or tropical grasses high in oxalate, such as: Setaria sphacelata, Cenchrus ciliaris (buffel grass), Brachiaria mutica (para grass), Digitaria decumbens (pangola grass), Pennisetum clandestinum (kikuyu grass), and Panicum spp. These are dangerous because oxalate binds calcium and makes it unavailable for absorption. The characteristic gross finding in horses is “big head” or bilateral enlargement of the maxilla and mandible.2 In captive birds with fibrous osteodystrophy, nutritional imbalances in calcium, phosphorus, and vitamin D are often compounded by inadequate amounts of unfiltered sunlight, which provides ultraviolet light required for birds to make vitamin D. The lighting conditions of the penguin in this case are unknown.
Contributing Institution:
University of Tennessee College of Veterinary Medicine
Department of Biomedical and Diagnostic Sciences
2407 River Drive, Room A205
Knoxville, TN 37996
https://vetmed.tennessee.edu/departments/Pages/biomedical_diagnostic_sciences.aspx
References:
- Adkesson MJ, Langan JN. Metabolic bone disease in juvenile Humboldt penguins (Spheniscus humboldti): investigation of ionized calcium, parathyroid hormone, and vitamin D3 as diagnostic parameters. J Zoo Wildl Med. 2007;38:85-92.
- Craig LE, Dittmer KE, Thompson KG. Bones and joints. In: Maxie, MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th Vol. 1. St. Louis, MO: Elsevier; 2016:74-80.
- Dacke CG. The parathyroids, calcitonin, and vitamin D. In: Whittow CG, ed. Sturkie’s Avian Physiology. 5th ed. San Diego Academic Press; 2000;473-477.