Signalment:
This monkey survived the bacterial challenge and was euthanized at the end of the experiment. The carcass was then submitted for a complete necropsy.
This experiment and the necropsy were performed under biosafety level 3 (BSL-3) biocontainment conditions. This research was conducted under an IACUC approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
This research was sponsored by the Joint Science and Technology Office (JSTO), [Project No. R.R.0001_07_RD_B].
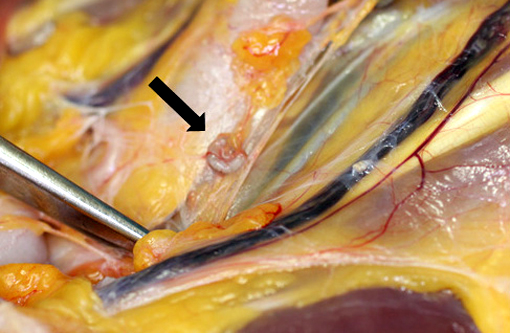
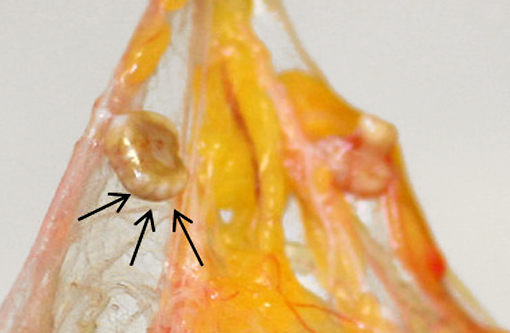
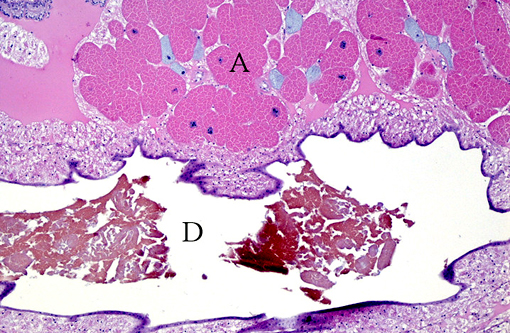
Gross Description:
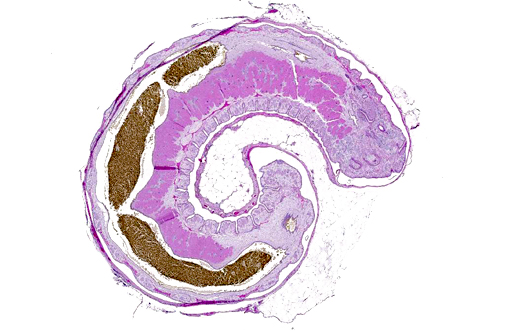
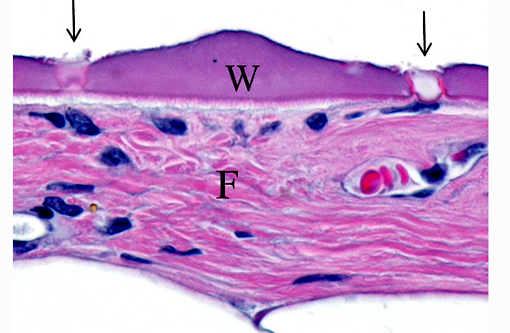
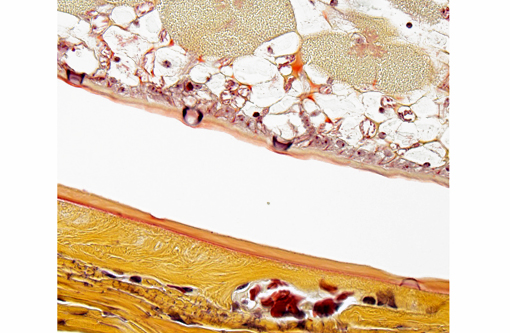
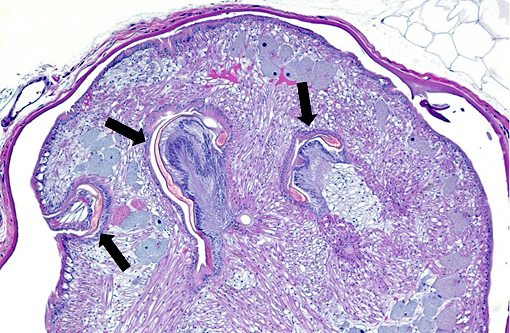
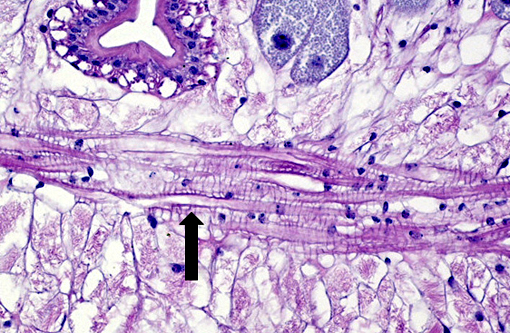
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Pentastomes are a unique group of animals, all of which are parasites. The adult parasites inhabit the respiratory tract of vertebrates. More than 100 species have been described and for approximately 90% of these, the definitive host is a carnivorous reptile.(4) However, there is one species, Linguatula serrate, where the adult parasites live in the nasal passages of dogs and other carnivores. Adults in the genus Linguatula have a shape that resembles a mammalian tongue; this is the basis for the genus name and for the common term of tongue worms for pentastomes in general.
Each pentastome has two pairs of cephalic hooks adjacent to its mouth. These hooks are used to assist in attachment and burrowing. Early researchers mistook these hooks to be four additional mouths; this error is the basis for the name of this group of parasites (i.e., penta stoma).(3)
Although a few pentastome species have a direct life cycle, the great majority of species utilize intermediate hosts.(4) Adult females lay eggs containing legged larvae that resemble mites.(1) The larvated eggs are passed into the environment either directly in respiratory secretions or are swallowed and passed in feces. Intermediate hosts become infected by ingestion of food or water contaminated with the eggs. Eggs hatch in the intestinal tract of the intermediate host and the larvae burrow through the intestinal wall. The larvae then develop into nymphs and encyst in the viscera and/or serous membranes of the intermediate host. After the intermediate host is consumed by the definitive host, the nymphs migrate to the respiratory tract of the final host and develop into adults.
A wide variety of animals (including humans) can serve as intermediate hosts for pentastomes. It is very difficult to accurately identify a pentastome species based solely on the morphology of nymphs.(5) In a report about the massive pentastome nymph infection of a stray dog from the southern United States, the species of pentastome was identified as Porocephalus crotali using a combination of gross parasite morphology and DNA analysis.(2)
The species of pentastome present in this monkey was not identified. However, the definitive host is most likely a snake or crocodilian that inhabited the native environment of this macaque. In a recent report about a massive pentastome infection of a cynomolgus macaque imported from China, the pentastome nymphs were identified as Armillifer agkistrodontis by DNA analysis.(5) Armillifer agkitrodontis adults occur in the lungs of pit vipers in SE Asia and the usual intermediate hosts are small rodents that serve as the prey base for the snakes; the monkey described in the case report was thought to be an accidental, dead-end host.(5)
The parasites present in this WSC case submission elicited mild fibrosis and very little inflammation by its primate host; this is typical, even in cases of massive pentastome infections.(2,5) However, fatal peritonitis has occasionally been associated with nymph penetration of the intestinal wall.(6) Dead nymphs cause intense inflammation and may eventually become mineralized.Â
In humans, three types of histologic lesions caused by pentastomid nymphs have been described.(7) The first lesion consists of an encysted and viable nymph surrounded by little to no inflammation (as in this monkey). In the second type of lesion, a dead nymph is surrounded by granulomatous and usually eosinophilic inflammation, encapsulated by concentric rings of fibrosis. In the third type of lesion, known as the granulomatous scar or cuticle granuloma, fibrous tissue surrounds a central mass of amorphous and/or mineralized material consisting of remnants of the long-dead parasite.Â
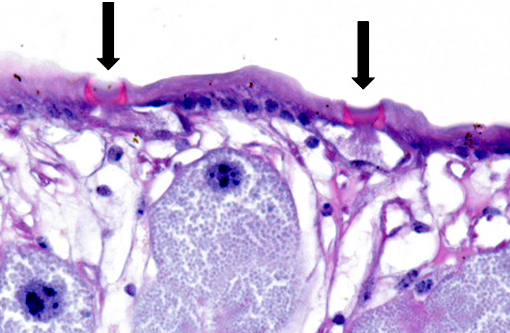
The cuticle of a pentastome often remains long after the rest of the dead parasite has degenerated. Finding parts of a cuticle with sclerotized openings within a focus of inflammation proves that the lesion was caused by a pentastome because no other parasites have sclerotized openings.(3) Visualization of the sclerotized openings can be greatly enhanced with a Movat pentachrome or Massons trichrome stain.(3,7) In this case, the cyst wall also has sclerotized openings. This means that the wall is composed of a molted cuticle from the parasite.
The taxonomic classification of pentastomes was a subject of debate for many years. It has been long-thought that they should be classified as arthropods or arthropod-like animals. Based on more recent DNA analyses, it is now generally accepted that pentastomes constitute a unique subclass (i.e., Pentastomida) of crustacean arthropods and are most closely related to the crustacean subclass Branchiura -- species of which are ectoparasites of fish and are commonly known as fish lice.(4)
Acknowledgement: The author wishes to thank Dr. Kathleen Cashman for providing photography assistance and the gross photographs.
Note: Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.Â
JPC Diagnosis:
Conference Comment:
Conference participants discussed the peculiarity of consistently observing the shed cuticle, or exuvia, lining the cystic cavity which encloses the pentastome. The exuvia is never observed floating freely, but rather always tightly adhered to tissue forming the cyst wall. This exuvia is from a previous nymph, and as nymphal stages undergo multiple molts before becoming a mature adult, it is possible the exuvia becomes a protective barrier from the immune defenses of the host. This is supported by the finding that intact cysts with viable nymphs are usually found with little or no adjacent cellular infiltration of the host tissue as antigenic compounds are largely sequestered.(7)
References:
1. Bowman DW, Lynn RC, Eberhard ML, Alcaraz A. Georgis Parasitology for Veterinarians. 8th ed. St. Louis, MO: Saunders; 2003.
2. Brookins MD, Wellehan JFX, Roberts JF, et al. Massive visceral pentastomiasis caused by Porocephalus crotali in a dog. Vet Path 2009; 46:460-463.
3. Gardiner CH, Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues., Washington, DC; Armed Forces Institute of Pathology; 2006.
4. Lavrov DV, Brown WM, Boore JL. Phylogenetic position of the Pentastomida and (pan)crustacean relationships. Proc R Soc Lond B. 2004; 271:537-544.
5. Matz-Rensing K, Lampe K, Rohde G, Kaup F-J. Massive visceral pentastomiasis in a long-tailed macaque an incidental finding. J Med Primatol. 2012;41(3):210-213.Â
6. Strait K, Else JG, Eberhard ML. Parasitic diseases of nonhuman primates. In: Abee CR, Mansfield K, Tardif S, Morris T, eds. Nonhuman Primates in Biomedical Research Volume 2: Diseases. 2nd ed. Boston, MA: Academic Press; 2012:271-272.
7. Tappe D, B+�-+ttner DW. Diagnosis of human visceral pentastomiasis. PLoS Negl Trop Dis. 2009; 3(2):e320. doi:10.1371/journal.pntd.0000320