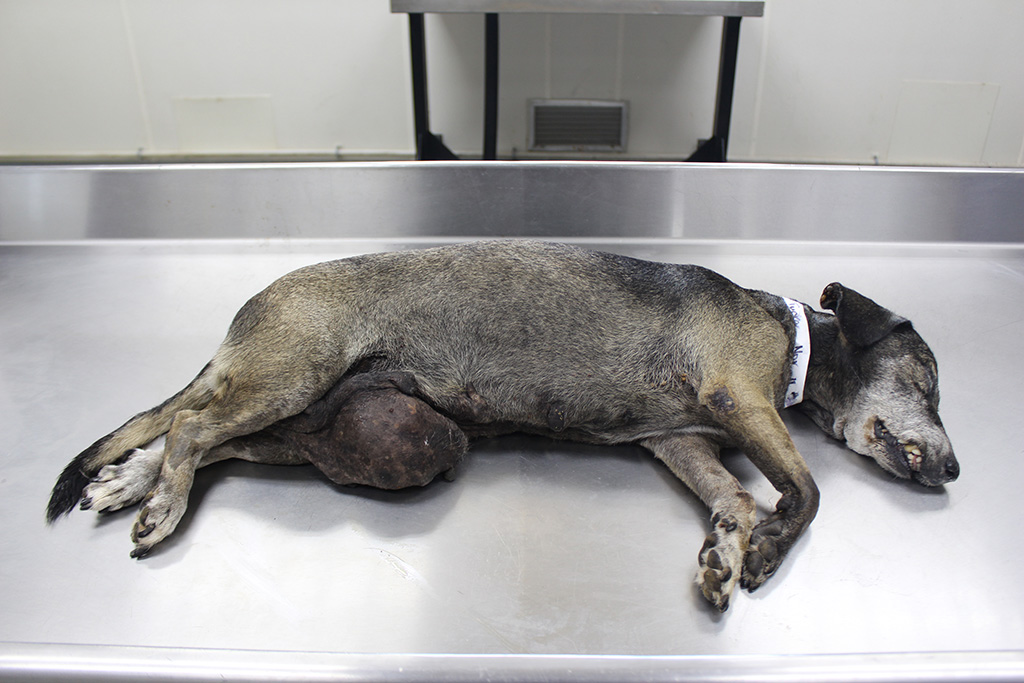
Signalment:
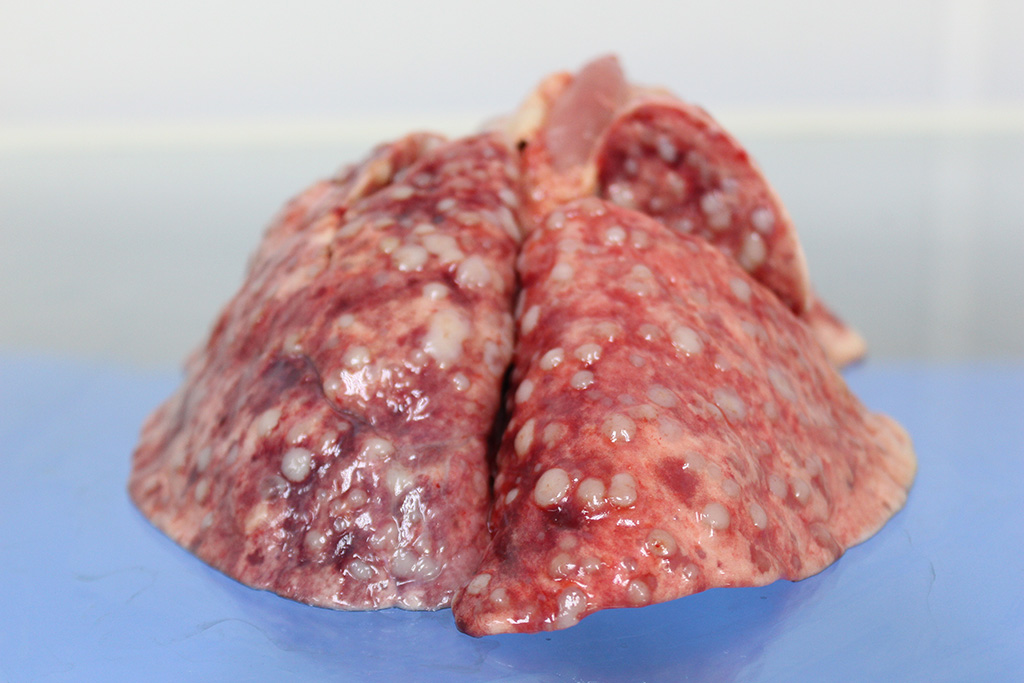
Gross Description:
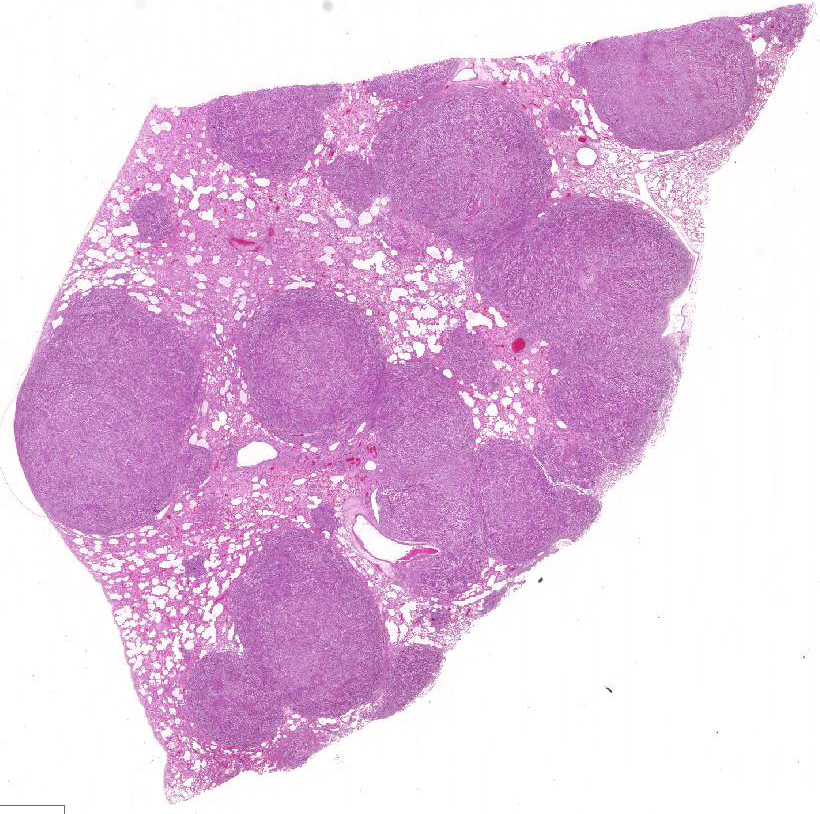
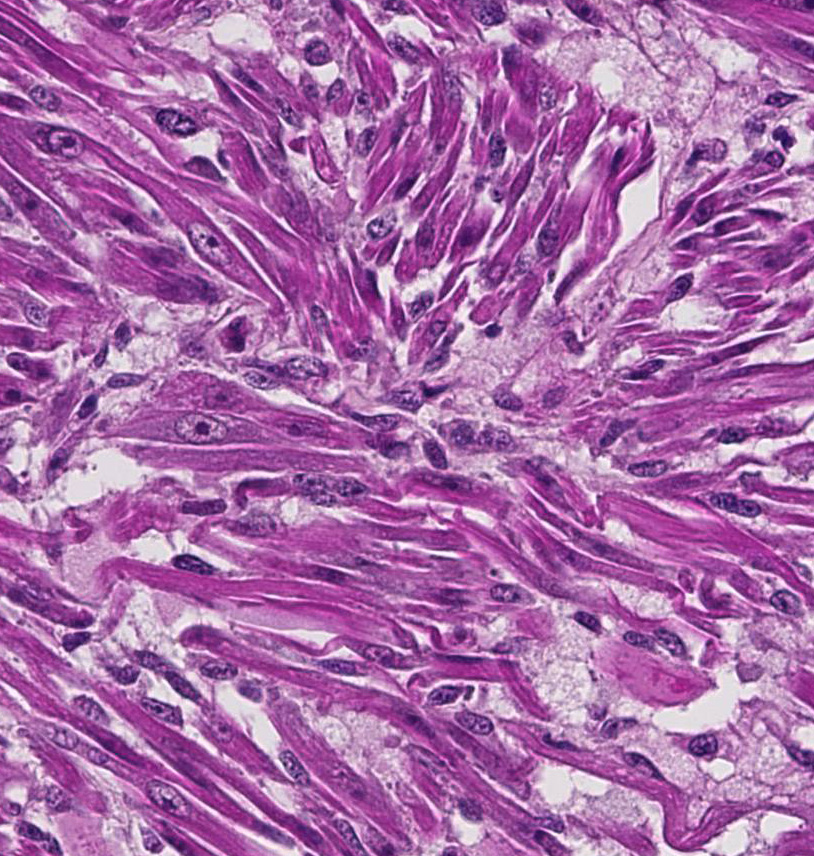
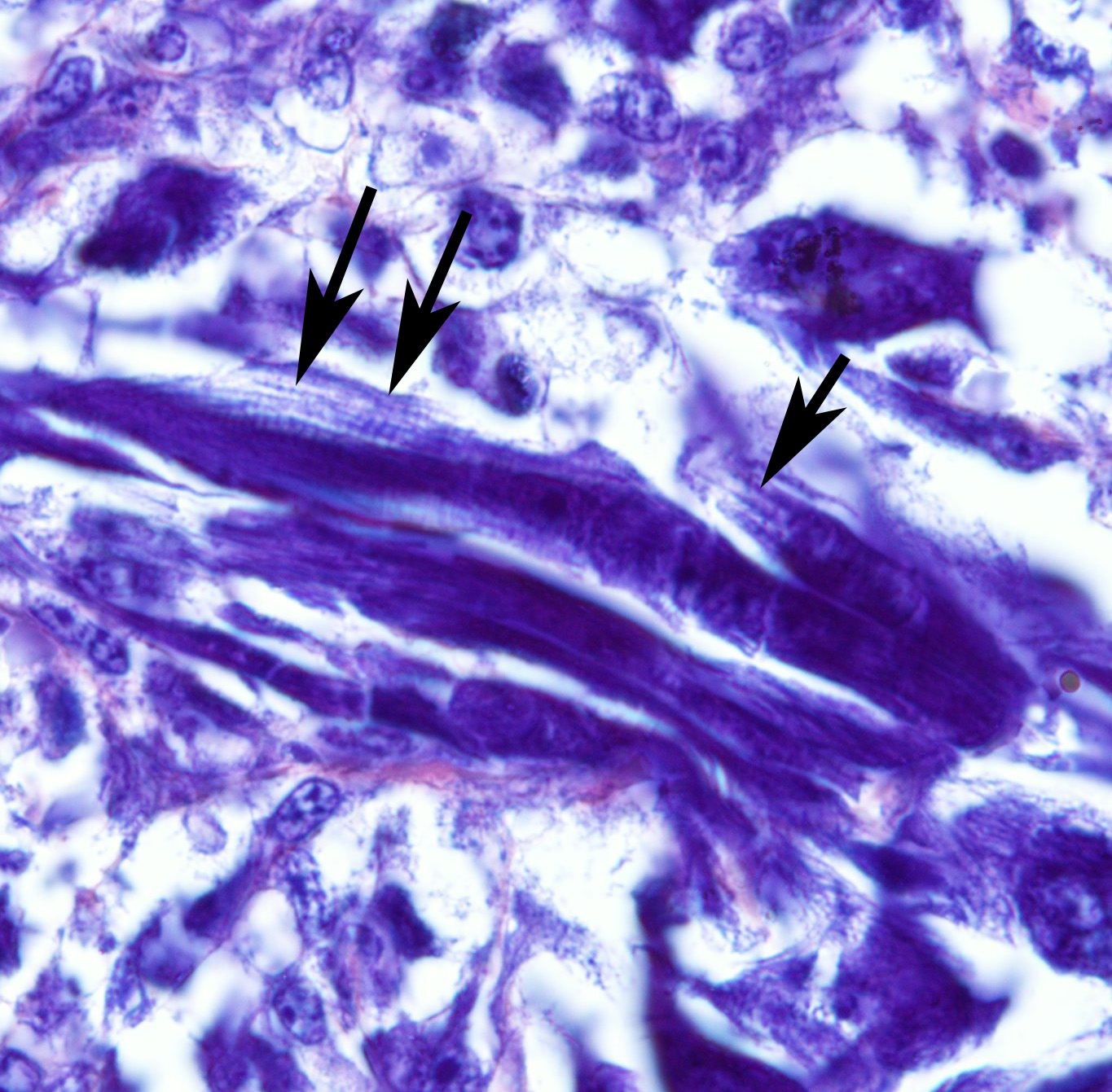
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In humans, embryonal rhabdomyosarcoma is the most common type, and predominantly affects children.7 Similarly, embryonal rhabdomyosarcoma is the most common type diagnosed in dogs, and usually involves dogs under two years of age. However, when excluding the botryoid type of embryonal rhabdomyosarcoma of the urinary bladder, approximately half of the reported cases of canine embryonal rhabdomyosarcoma occur in adult dogs, as observed in this case.1 In humans, embryonal rhabdomyosarcoma is considered an intermediately aggressive variant of rhabdomyosarcoma. Canine rhabdomyosarcoma, in general, is considered an aggressive malignancy having a metastatic rate which appears to approximate that of grade three soft tissue sarcomas. However, there is too little data to make conclusions regarding the prognostic significance of histological subtypes in dogs. The present case would appear to comprise the first report of histologically-confirmed metastasis of embryonal rhabdomyosarcoma in an adult dog (>2 years), but appears to be of intermediate malignancy since the dog survived over nine months following clinical diagnosis of the mammary mass.
Rhabdomyosarcoma has occasionally been reported in dogs to emerge from organs lacking striated muscle, including skull,4 meninges,6greater omentum,8 gingiva,9 spleen, perirenal, and mammary gland.2 In such instances, the neoplasm is thought to arise from stem cells capable of myogenic differentiation. In the present case, the large mammary mass is presumed to be the primary tumor because it was the largest of all masses and was first recognized clinically. Mammary rhabdomyosarcoma is very rare in humans and usually affects adolescent girls, is metastatic rather than primary breast tumor, and is alveolar subtype.5 Similar to the present case, the other reported case of canine mammary rhabdomyosarcoma involved an adult female, and was also of the embryonal subtype.2
JPC Diagnosis:
Conference Comment:
As mentioned by the contributor, skeletal muscle neoplasms often arise in areas where skeletal muscle is not normally present. Common locations in the canine are the larynx and urinary bladder.3 Laryngeal rhabdomyomas are formally known as laryngeal oncocytomas due to their characteristic eosinophilic, granular, PASpositive cytoplasm. The name was changed to rhabdomyoma due to the presence of myofilaments with Z-bands on transmission electron microscopy (TEM), and positive immune-reactivity for muscle markers.3 In the urinary bladder, RMS is also known as botryoid rhabdomyosarcoma. This most commonly occurs in young (<2 years), large breed bitches, with Saint Bernard dogs overrepresented. Grossly, these botryoid neoplasms occur at the trigone and cause urinary obstruction.3 Interestingly, they have also been associated with hypertrophic osteopathy in dogs.3 In pigs, cardiac rhabdomyomas are benign incidental findings and are thought to be Purkinje cell origin due to expression of protein gene product (PGP) 9.5, a Purkinje fiber marker.3
Almost all RMSs are typically aggressive with local infiltration and distant metastasis to other muscles, liver, spleen, and the lung.3 In this case, in addition to the lung and adjacent mammary tissue, there is metastasis to the kidneys, spleen, liver, epicardium, and myocardium.
RMS in humans and veterinary species has been classified into different categories depending on the degree of differentiation towards skeletal muscle:
Embryonal: Divided based on cell morphology: myotubular, myoblastic, spindle cell, and botryoid. Embryonal RMS is typically encountered in animals <2 years old, and occur on the face, skull, masticatory muscle, oropharynx, trachea, axilla, scapula, perirenal, tongue, flank, leg, mammary gland, and hard palate. Botryoid RMS occurs in the urinary bladder and uterus. 1,2,3 The mammary gland is theorized to be the primary tumor location in this case.
- o Myotubular: Multinucleated strap cells and racquet cells with cross-striations.
- o Myoblastic: Most common form, and is composed of small round cells with abundant eosinophilic cytoplasm.
- o Spindle cell: Low cellularity and arranged in a storiform pattern.
- o Botryoid: Submucosal location with mixed round and myotubular cells. The term botryoid is due to grapelike appearance grossly.1,3
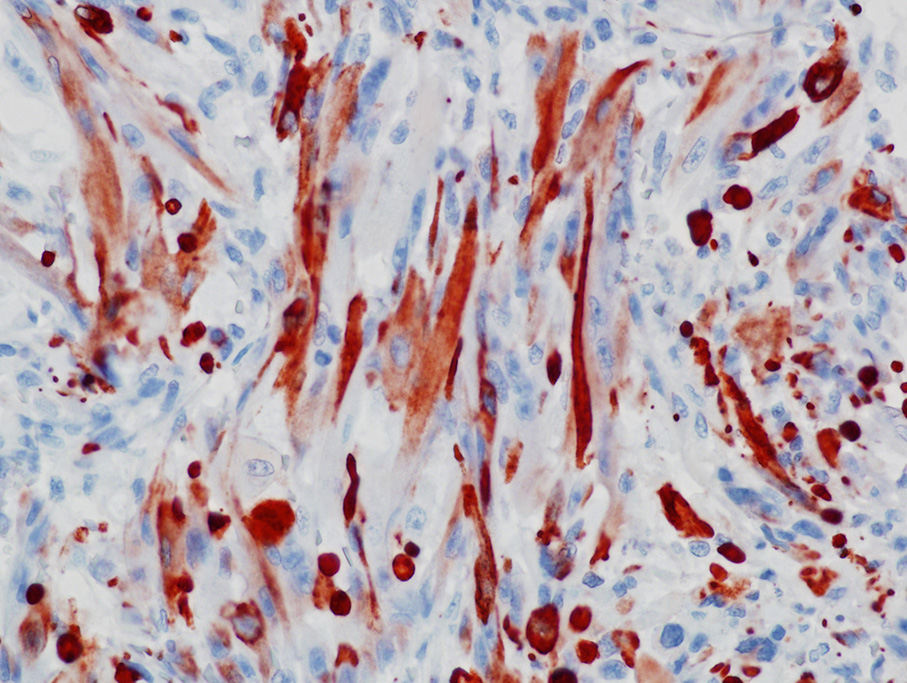
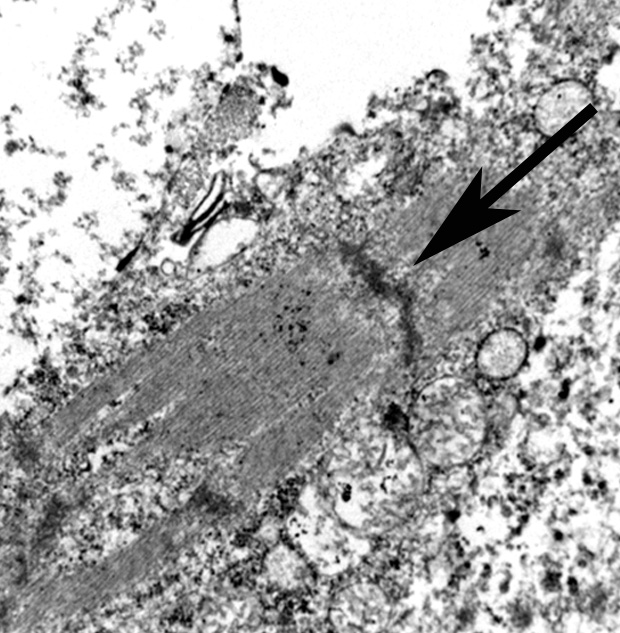
The use of immunohistochemistry (IHC) to identify vimentin, desmin, α-actins, myoglobin, myogenin, and MyoD1 in conjunction with TEM to identify of sarcomeric structures, Z-bands, and large mitochondria, can also be useful in the diagnosis RMS.1,2,3
References:
2. Cooper BJ, Valentine BA: Tumors of muscle. In: Meuten DJ, ed. Tumors in Domestic Animals. Fourth Edition ed. Ames, Iowa: Iowa State Press; 2002:319-364.
3. Cooper BJ, Valentine BA. Muscle and tendon. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 6th ed., Vol. 1. Saunders Elsevier, Philadelphia, PA, 2016:241-243.
4. Da Roza MR, De Amorim RF, Carneiro FP, Benatto N, Barriviera M, Miguel MC: Aggressive spindle cell rhabdomyosarcoma in an 11- month-old boxer dog. J Vet Med Sci. 2010; 72(10):1363-1366.
5. Hays DM, Donaldson SS, Shimada H, Crist WM, Newton WA, Jr., Andrassy RJ, et al.: Primary and metastatic rhabdomyosarcoma in the breast: neoplasms of adolescent females, a report from the Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997; 29(3):181-189.
6. Illanes OG: Juvenile parameningeal rhabdomyosarcoma in a dog causing unilateral denervation atrophy of masticatory muscles. J Comp Pathol. 2002; 126(4):303-307.
7. Newton WA, Gehan EA, Webber BL, Marsden HB, van Unnik AJM, Hamoudi AB, et al.: Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification-an intergroup rhabdomyosarcoma study. Cancer. 1995; 76(6):1073-1085.
8. Sarnelli R, Grassi F, Romagnoli S: Alveolar rhabdomyosarcoma of the greater omentum in a dog. Vet Pathol. 1994; 31(4):473-475.
9. Snyder LA, Michael H: Alveolar rhabdomyosarcoma in a juvenile labrador retriever: case report and literature review. J Am Anim Hosp Assoc. 2011; 47(6):443-446.