CASE 1: MS1401949 (JPC 4052879-00)
Signalment:
3 month old male C57BL/6N mouse
History:
A 3 month old male C57BL/6N mouse received total body irradiation of 900 rads and subsequently received 5 x 106 syngeneic bone marrow cells. Two months later the mouse was infected with 1.5x104 Listeria monocytogenes IV via tail vein injection. The mouse became morbid within days and was submitted for necropsy following euthanasia.
Gross Pathology:
The mouse was thin and contained a small amount of body fat. The spleen was of normal size but moderately pale and mottled. The liver was mildly enlarged uniformly. The heart, lungs, kidneys, GI tract, testes and brain appeared normal.
Laboratory results:
A sample of spleen was collected for bacterial culture and yielded a pure growth of Listeria monocytogenes.
Microscopic description:
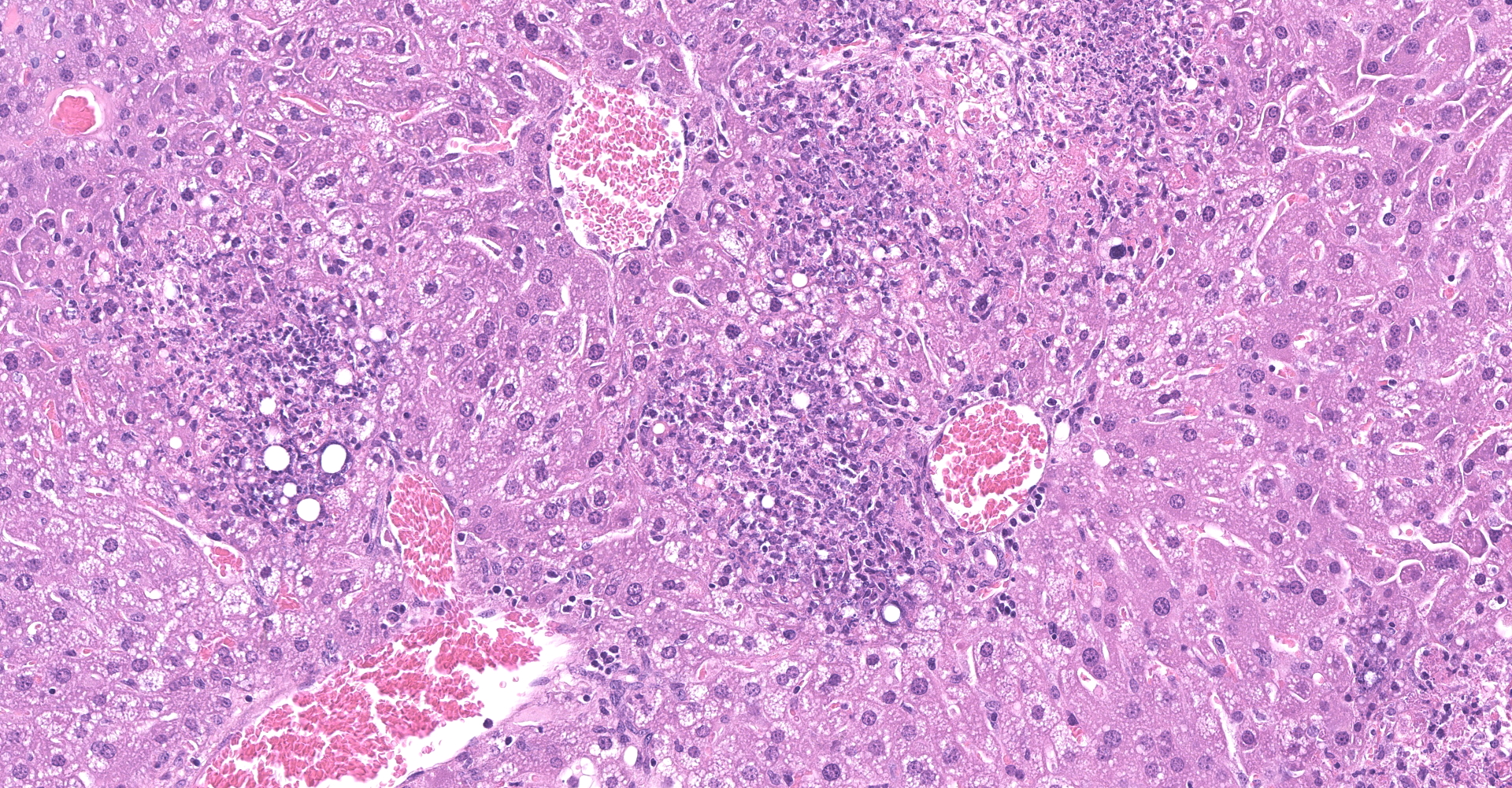
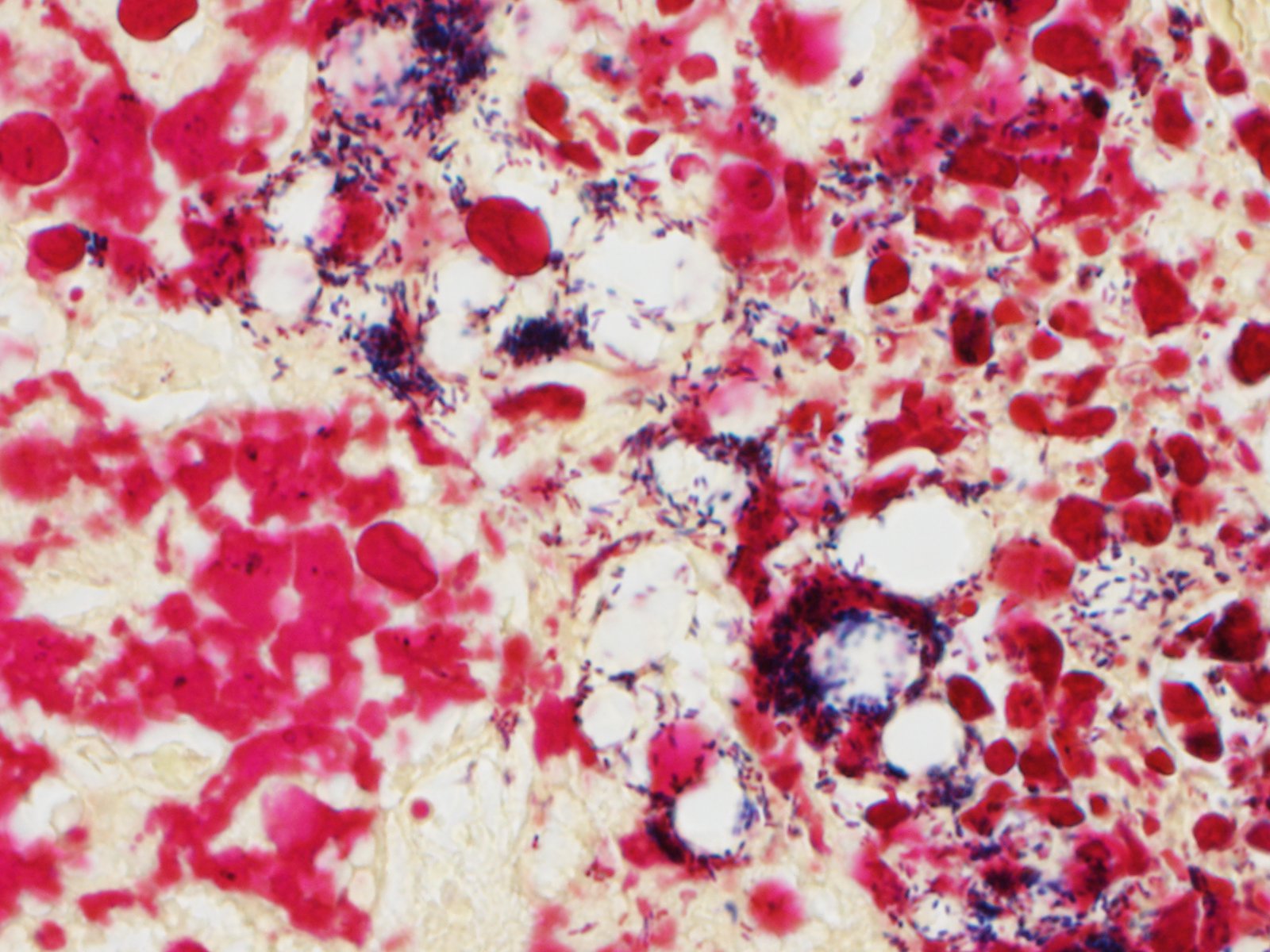
Liver - There is multifocal, random acute coagulative necrosis throughout the section. Small numbers of degenerative neutrophils are present along the margins of the necrotic foci. Intracellular bacilli are evident within hepatocytes at the margins of the necrotic foci. Mild to moderate intracellular lipidosis is present with the lipidosis being more prominent in hepatocytes at the margins of the necrotic foci.
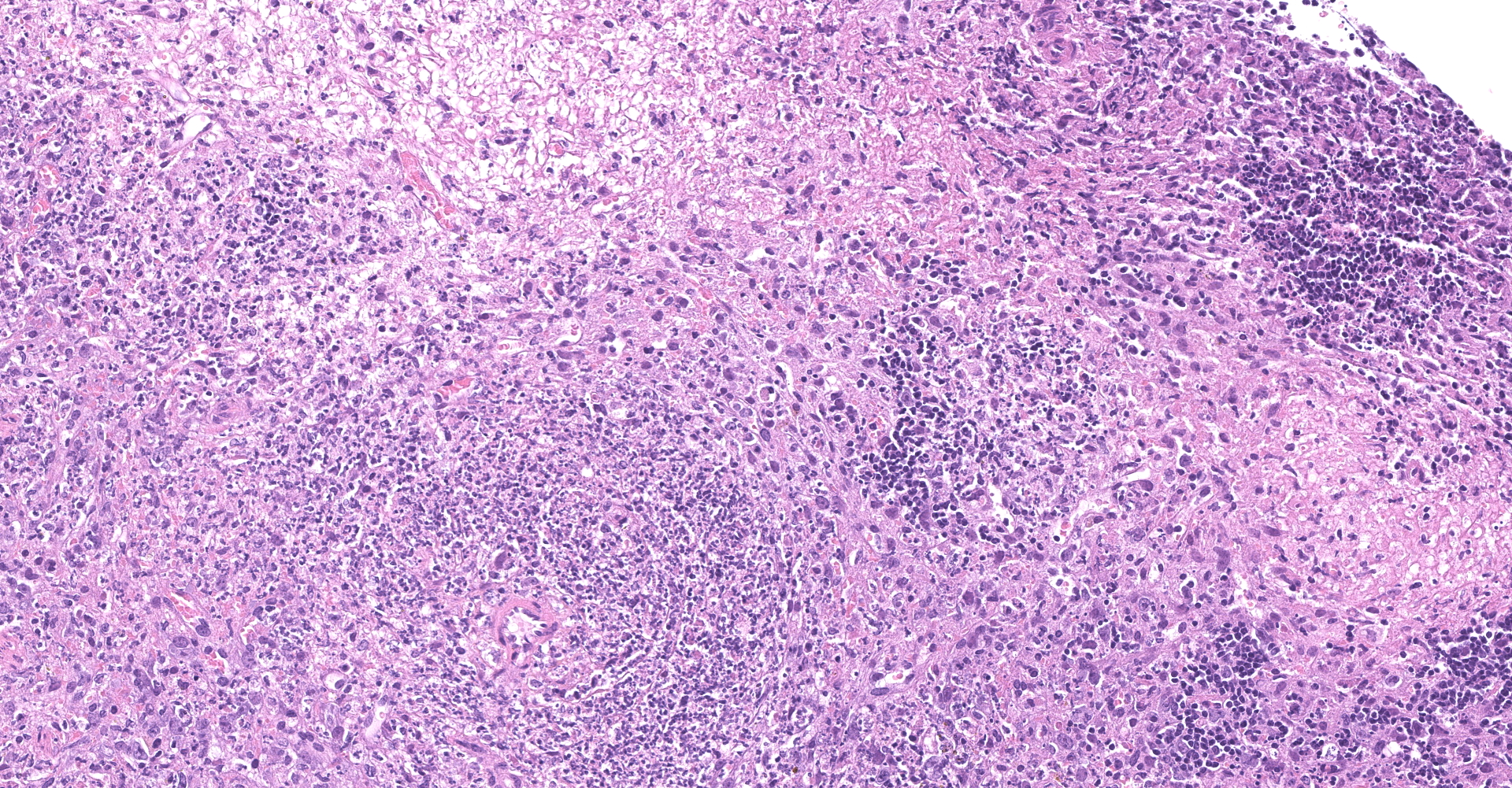
Spleen - Multiple foci of necrosis are evident which appear to correspond primarily with lymphoid follicles. Degenerative neutrophils are evident adjacent to necrotic foci, within sinusoids and the capsule of the spleen. Short gram positive bacilli consistent with Listeria monocytogenes are evident within the spleen. Minimal extramedullary hematopoiesis is noted in the spleen.
Lesions were not noted in other organs/tissues.
Contributor's morphologic diagnosis:
Spleen - splenitis, necrotizing, acute, severe
Liver - hepatitis, multifocal, necrotizing, acute, severe
Contributor's comment:
Listeria monocytogenes is a gram positive, facultative, intracellular bacillus. It is a ubiquitous agent found in the soil which can survive in the environment for months or longer. The agent is one of the leading causes of food- borne disease.
Listeria was first discovered by E.G.D. Murray in 1926 associated with outbreaks affecting rabbits and guinea pigs. The first human case was reported in 1929, with the first human epidemic associated with ingestion of contaminated food in 1983.2 The organism grows best at -18° to 10° C. The ability to grow at these temperatures allows for transmission from foodstuffs which have been properly refrigerated. The agent has been isolated from raw and undercooked meat and poultry, processed meats, vegetables, soft cheeses, ice cream and unpasteurized milk. Vegetables can be contaminated from soil or manure used as fertilizer.8
Human infections in the United States occur at approximately 2500 per year with a mortality rate of 20 to 30%. High risk groups include the elderly, infants, pregnant women, fetuses, and immunocompromised individuals. Infection of humans and animals is typically via ingestion of contaminated foodstuffs. The bacterium typically causes a self-limiting gastroenteritis and can reside in the intestinal tract for long periods without inducing disease.4
Infection beyond the gastrointestinal tract generally leads to one of three significant clinical syndromes - meningitis/meningoencephalitis, septicemia, and third trimester abortion. In humans, disease is typically manifested as a meningitis and/or meningoencephalitis. In animals listeriosis is primarily a disease of ruminants affecting sheep, cattle and goats and has also been reported in llamas, alpacas, deer, reindeer, antelope, water buffalo, moose and captive giraffe. Infection is often associated with ingestion of spoiled silage. The disease in ruminants is typically a CNS disorder with suppurative meningitis or meningoencephalitis and cerebral microabscesses, with clinical signs including circling, ataxia, opisthotonos and possible cranial nerve paralysis. The CNS signs are due to centripetal migration along cranial nerves particularly the trigeminal nerve, with lesions commonly involving the brainstem, with gross evidence of meningitis along the ventral midbrain, pons and medulla oblongata. In ruminants, particularly sheep, infection may also be caused by L. ivanovii.6 In monogastric animals the disease is primarily a septicemia and has been reported in rodents and lagomorphs and rarely in dogs, cats, horse and swine. In nonhuman primates disease is rare and has been reported in chimpanzees, baboons, and Celebese black apes. Disease has also been reported in avian species including chickens, turkeys, ducks, geese, pigeons, canaries, parrots, eagles, owls and partridges.5 Mice are susceptible to experimental infection but are relatively resistant to infection orally and require administration by a parenteral route. Susceptibility to infection varies by strain with A/J and BALB/c being more sensitive to infection than C57BL/6.5
Infection follows invasion of the intestinal epithelium with replication in the lamina propria and extension to the mesenteric lymph nodes with dissemination to the liver and spleen. Secondary viremia can lead to dissemination to the cranial nerves and the meninges and to the placenta in pregnant females. Invasion of epithelial cells is receptor mediated and involves expression of internalin genes in1A, in1B, and ami on the surface of the bacterium which bind E-cadherin inducing adhesion. Following multiplication in the cytosol the bacteria induce actin polymerization to aid in direct transfer to adjacent cells. Listeria monocytogenes can enter dendritic cells and histiocytes via phagocytosis. Listeriolysin O, a secreted cholesterol induced cytolysin, mediates escape from the phagocytic vacuoles into the cytosol. Protection against the bacterium is mediated primarily by interferon gamma produced by NK and T cells.5
Contributing Institution:
National Institutes of Health
Division of Veterinary Resources
Diagnostic and Research Services Branch
JPC diagnosis:
1. Liver: Hepatitis, necrotizing, random, multifocal, marked, with numerous intracellular and extracellular bacilli.
2. Spleen: Splenitis, necrotizing, multifocal to coalescing, severe, with thrombosis and lymphocytolysis.
JPC comment:
The contributor provides an excellent summary of Listeria monocytogenes. Because this bacterium causes significant disease in human populations, as well as animal populations, there is utility in tracking outbreaks and potentially drawing conclusions about patterns of infection. ProMED is an organization that aggregates official and unofficial data and provides resources and updates regarding disease outbreaks for a number of entities. Importantly, this data aggregation is publicly available, which can help inform the allocation of resources, evaluation of intervention efficacy, and novel aspects of the disease. A study of human L. monocytogenes cases from 1996-2018 covered the largest outbreak recorded, in South Africa, where there were more than 1000 laboratory confirmed cases, and more than 200 deaths. In the analyzed time period, there was also a marked increase in outbreaks involving novel foods not previously associated with L. monocytogenes. The increases are hypothesized to be due to changing food distribution methods and government regulation, the complexity of the global food distribution system, and the widespread consumption of ready to eat products.3
While to a great degree, L. monocytogenes is considered one entity, in reality, there are four evolutionary lineages, 13 serotypes, and four PCR groups within the species. Using multilocus sequence typing (MLST), there are further subdivisions into clones. These distinctions come with varying pathogenicity, and variable expression of virulence factors. In addition to In1A (listeriolysin S cluster) and genes responsible for synthesis of teichoic acid, a collection of six genes that encode a cellobiose-family PTS system, which impacts sugar and carbon metabolism and confers increased neurovirulence and maternal-neonatal infection virulence. The collection of six genes has a proposed name of Listeria pathogenicity island 4 (LIPI-4). Other clones of L. monocytogenes lack LIPI-4, but are capable of causing disease, indicating that additional factors contributing to their virulence remain to be discovered.7
Investigation into extracellular vesicles secreted by Gram positive bacteria was neglected until recently, as it was erroneously considered unlikely given their thick peptidoglycan cell walls. However, biologically active extracellular vesicles have now been described for a variety of fungi and Gram positive bacteria, in addition to the previous body of knowledge for Gram negative bacteria. L. monocytogenes secretes vesicles 20-200 nm in diameter containing listeriolysin O and phosphatidylinositol-specific phospholipase C. When tested, cell-free preparations of extracellular vesicles were toxic to murine macrophage cells, helping L. monocytogenes to survive intracellularly within hosts. By packaging virulence factors in a vesicle, it allows for concentrated delivery, without dilution as a function of distance.1
The moderator emphasized that while L. monocytogenes is relatively uncommon in mice, it is associated with abortion in rabbits. Other clinical manifestations include gastroenteritis, sepsis, and meningoencephalitis. As illustrated in this case, the experimental inoculation was performed as an intravenous injection, which has a higher degree of reproducibility in experimental models than oral infection.
References:
1. Coelho C, Brown L, Maryam M, et al. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J. Biol. Chem. 2019;294(4):1202-1217.
2. Cossart P. Listeriology (1926-2007): the rise of a model pathogen. Microbes and Infection. 2007; 9 (10): 1143-6.
3. Desai AN, Anyoha A, Madoff LC, Lassmann B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. International Journal of Infectious Disease. 2019;84:48-53.
4. Lecuit, M. Human listeriosis and animal models. Microbes and Infection. 2007; 9: 1216-1225.
5. D'Orazio S.E.F. Animal models for oral transmission of Listeria monocytogenes. Front. Cell. Infect. Microbiol. 2014; 11:4 15.
6. Hoelzer K. Pouillot R and Dennis S. Animal models of listeriosis: a comparative review of the current state of the art and lessons learned. Veterinary Research. 2012; 43:18.
7. Maury MM, Tsai YH, Charlier C, et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet. 2016;48(3):308-313.
8. Ramaswamy V, Cresence V, Rejitha, J, et. al. Listeria-review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007; 40:4-13.