WSC 2023-2024, Conference 23, Case 4
Signalment:
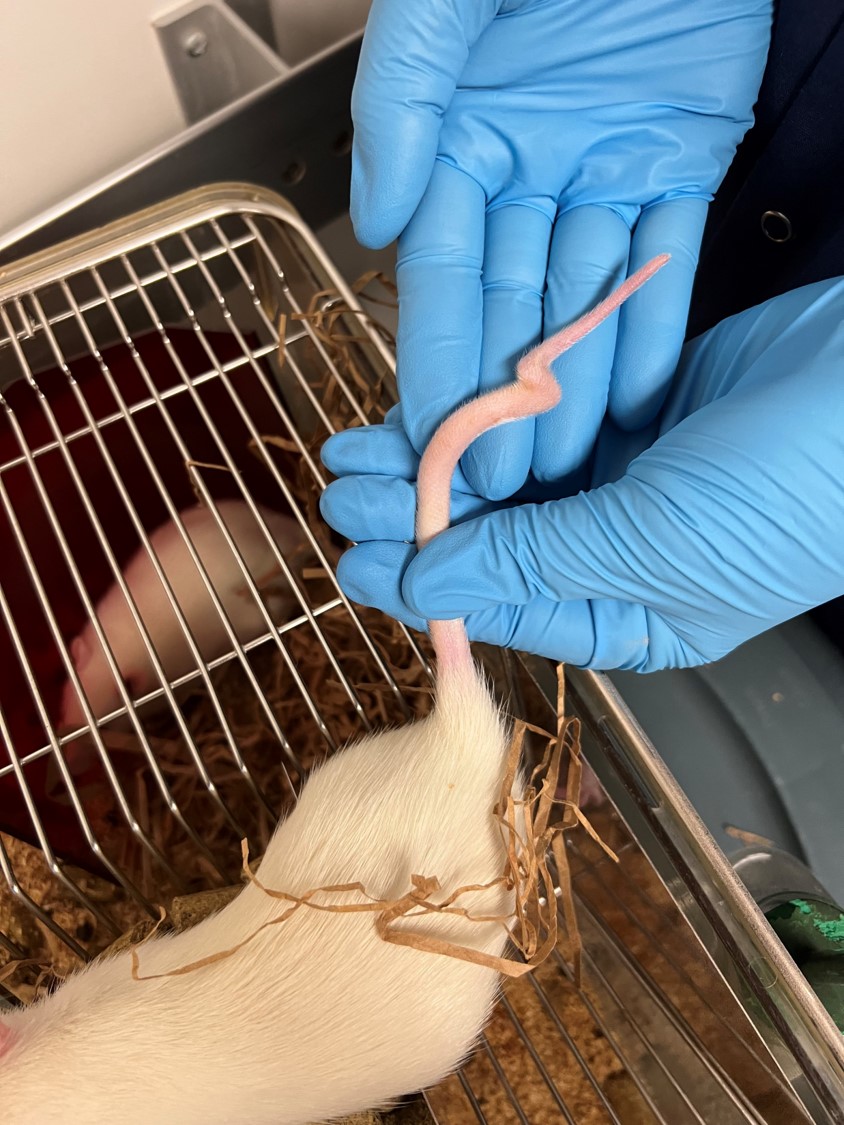
3-month old male Sprague Dawley rat (Rattus norvegicus).
History:
The mother of this rat was injected with valproic acid at 12 days gestation (animal model of autism). All of the pups in that litter had kinks in their tails.
Gross Pathology:
A 12 cm amputated tail was submitted in formalin. Three centimeters proximal to the tip, the tail is bent at an angle of approximately 270 degrees with a subtler bend 1 cm from the tip.
Microscopic Description:
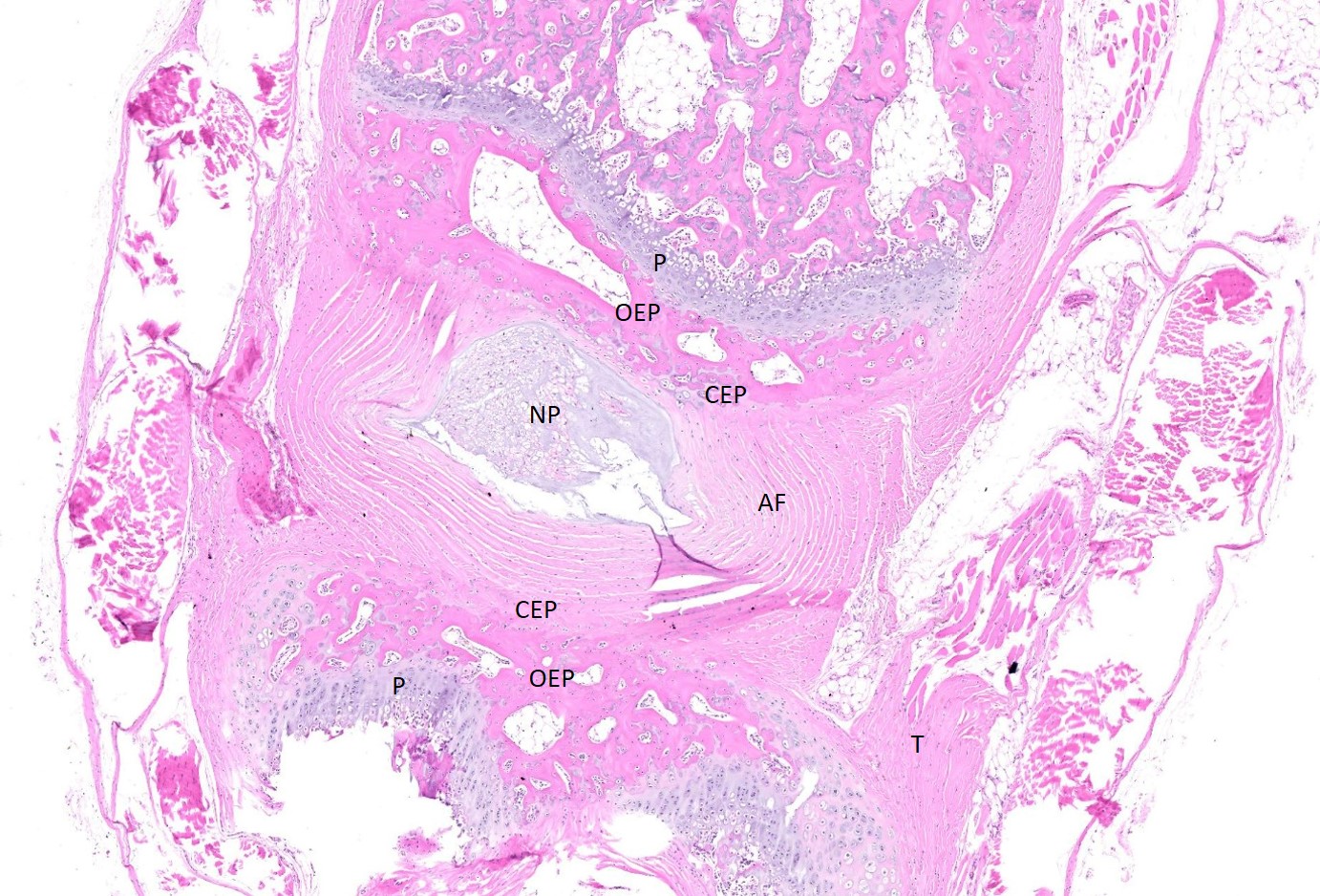
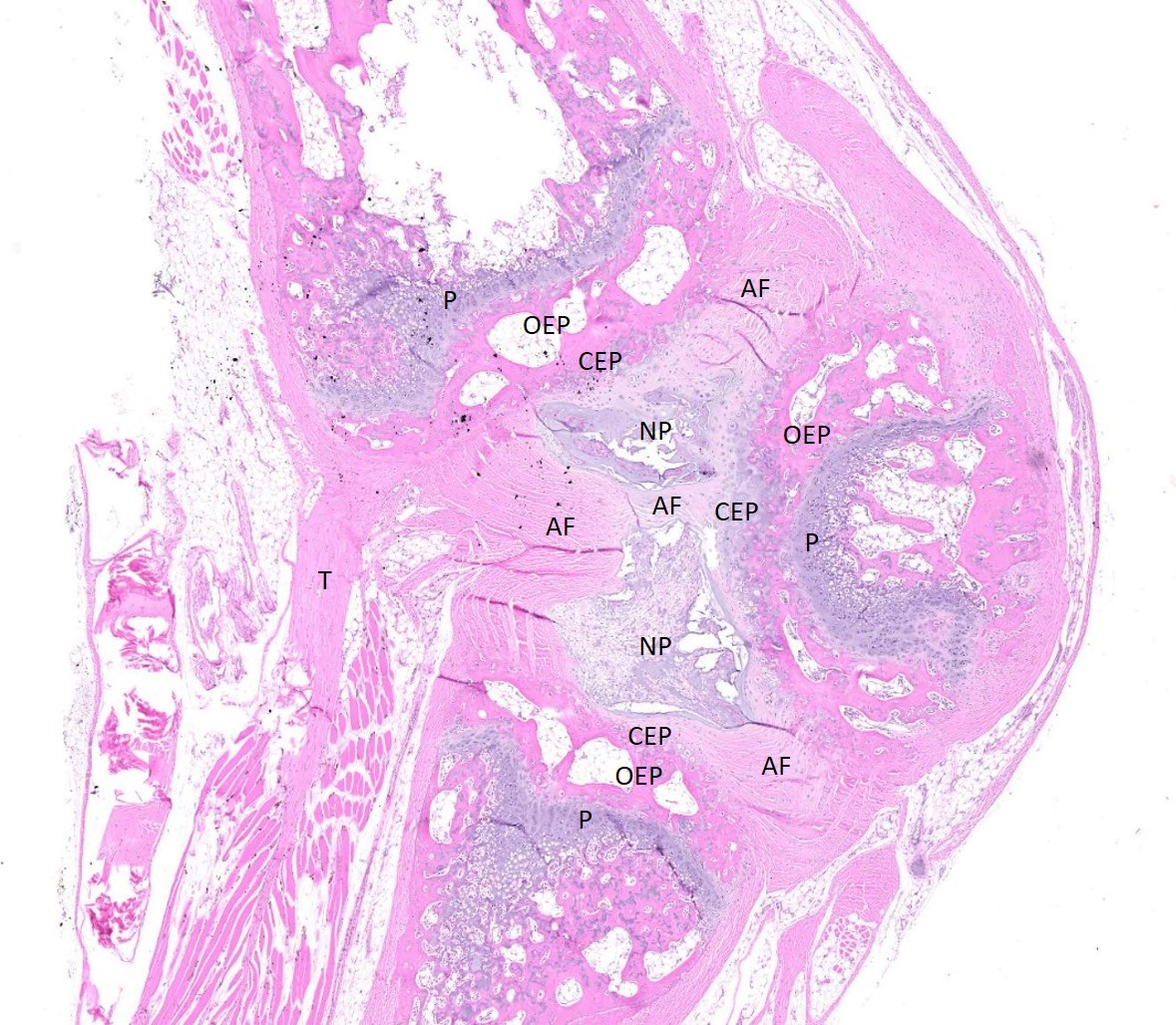
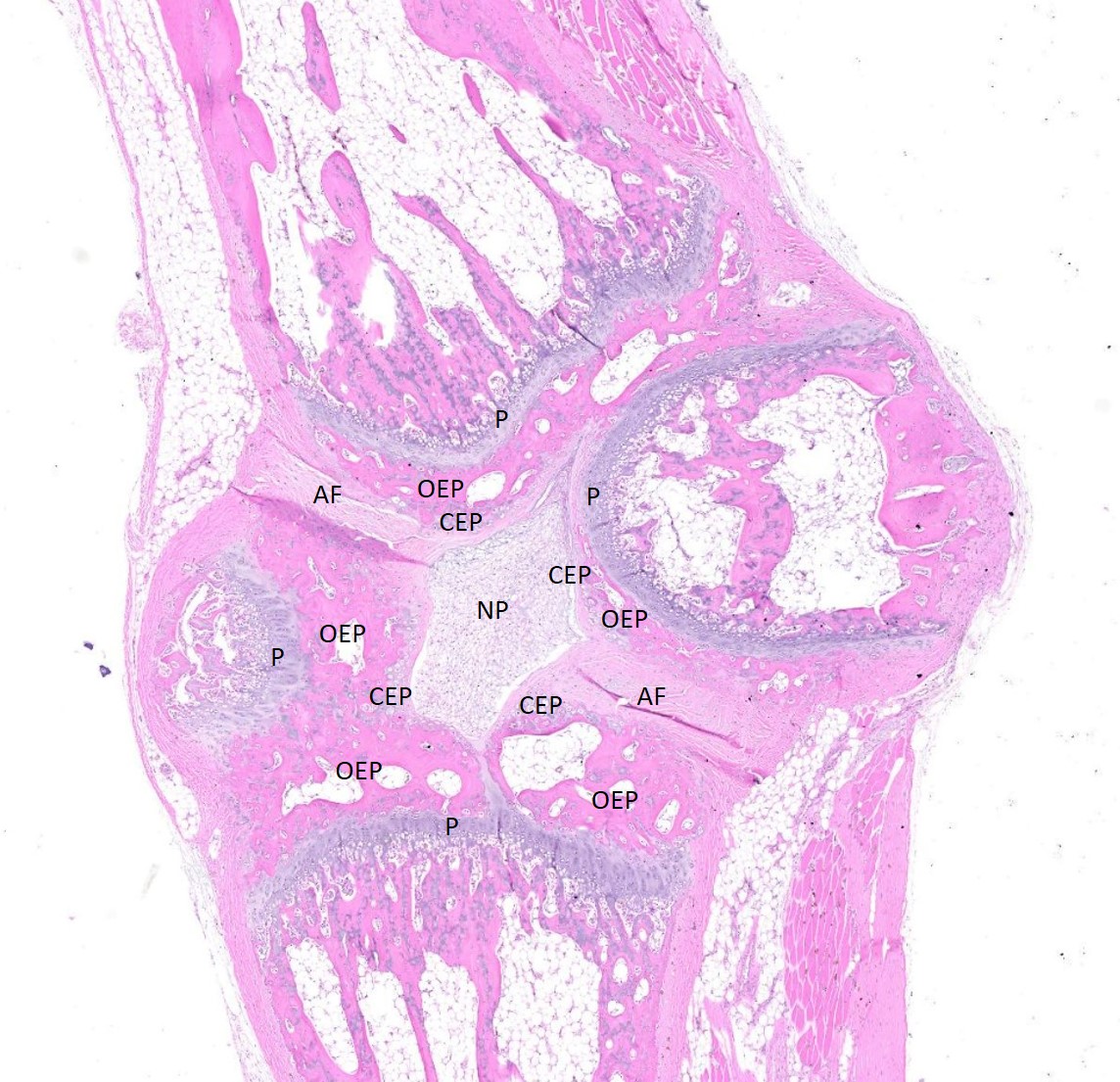
Examined are three longitudinal sections of the tail (skin removed). One section is composed mostly of muscle. In the other sections, two normal caudal vertebrae flank a malformed vertebra. In the middle section, the linear arrangement of the caudal vertebra is distorted by a small, eccentric, round to wedge-shaped bone containing a curved physis with somewhat disordered maturation of chondrocytes. The direction of chondrocyte maturation is oriented away from the midline of the tail. Medial to the physis is a layer of woven bone that is thicker at the periphery and thinner in the middle (osseous endplate) which blends with a layer of cartilage (cartilaginous end plate). Connecting to the periphery of the osseous endplate are laminar bands of collagen and fibroblasts which also connect to the adjacent vertebral body osseous endplates (annulus fibrosus). The annulus fibrosus also connects the osseous endplates of the cranial and caudal vertebrae and surrounds two foci of chondrocytes, amorphous amphophilic material (proteoglycans), and large vacuolated cells (nucleus pulposus cells). These two foci are separated by a band of the annulus fibrosus which connects to the center of the cartilaginous endplate of the abnormal vertebra. The changes in the other section are similar, except there are bilateral abnormal vertebral segments which are connected to one of the adjacent vertebrae by woven bone and separated from the other adjacent vertebra by a band of annulus fibrosus. The two bony segments are separated by a central pocket of nucleus pulposus cells and proteoglycans. The physis of one of the adjacent vertebrae blends with the central nucleus pulposus, bisecting the osseous and cartilaginous end plates.
Contributor’s Morphologic Diagnosis:
Caudal vertebrae: Multifocal vertebral malformations (hemivertebra and butterfly vertebra).
Contributor’s Comment:
The vertebrate tail is made up of between 2 (Manx cat) and 49 (long-tailed pangolin) caudal (or coccygeal) vertebrae. In tailless animals, the greatly reduced caudal vertebrae are fused to form the coccyx. The cranial-most caudal vertebrae have a well-developed vertebral arch with spinous and transverse processes. The vertebral canal contains the filium terminale and a variable number of caudal spinal nerve roots which exit the canal through the corresponding intervertebral foramina. The vertebrae toward the tip of the tail have a much simpler, cylindrical shape.
The intervertebral disc forms a fibrocartilaginous joint between adjacent caudal vertebrae. The intervertebral disc consists of a central nucleus pulposus surrounded by a tough annulus fibrosus. The nucleus pulposus is composed of water, type II collagen, proteoglycans, and nucleus pulposus cells. The large, vacuolated nucleus pulposus cells of young animals, similar to the notochordal cells (physaliferous cells) from which the disc is derived, are replaced by smaller, chondrocyte-like cells in adults.2 The annulus fibrosus is composed of type I collagen and fibrocartilage arranged in laminae that attach to the osseous endplates of two adjacent vertebral bodies and provide resistance to tensile forces on the spine. The osseous endplate has a strong, solid, peripheral epiphyseal rim and a central portion made of thin, porous bone.11 The intervertebral disc is separated from the cortical bone of the vertebral endplate by a thin layer of hyaline cartilage, type II collagen, and proteoglycans called the cartilaginous endplate.8 The dorsal and ventral boundaries of the intervertebral disc are the dorsal and ventral longitudinal ligaments.
Tail kinks can be caused by fracture with malunion or by congenital malformations. Congenital malformations can be further divided into genetic diseases and teratogenic effects during development of the vertebral column. Genetic tail kinks are common in dogs and cats, especially in dog breeds such as Bulldogs, Boston Terriers, and Pugs.3 Embryologic development of the vertebral column begins with segmentation of the paraxial mesoderm on each side of the neural tube into somites. Next, each somite is divided into a ventral sclerotome, the precursor for the vertebrae, and a dorsal dermomyotome, which develops into the skeletal muscle and dermis of the back. The ventral sclerotome subunits fuse in cranial-caudal pairs as well as across the midline to form the vertebral body and the vertebral arch which encloses the developing spinal cord. Development and chondrification of the vertebrae precedes in an cranial to caudal direction, while the progression of ossification is less orderly.1
Congenital vertebral malformations have been classified into either failure of segmentation, where the mesoderm does not properly divide into somites, leading to the appearance of vertebral fusion, or failure of vertebral formation, where elements of an individual vertebra do not form, leading to abnormally shaped vertebrae (wedge, hemi-, or butterfly vertebrae). Either type of deformity can lead to scoliosis (lateral deviation of the vertebral column) or kyphosis (dorsoventral deviation of the vertebral column). These defects have been classified based on which part (dorsal, lateral, or median) of the developing vertebra is deficient and whether the deficiency is complete (aplasia; hemivertebra) or incomplete (hypoplasia; wedge vertebra).3 A butterfly vertebra is caused by failure of the two lateral portions of the sclerotome to fuse across midline.5 These malformations can be further classified as segmented (separated from the adjacent vertebrae by intervertebral discs), nonsegmented (attached to the adjacent vertebrae by bone), or semi-segmented (attached to one adjacent vertebra by bone and separated from the other by an intervertebral disc). A typical vertebra has three centers of ossification (one in the body and one on each side of the arch) and four physes (one at each endplate and one on each side where the vertebral arch joins the body).1 Since most caudal vertebrae lack arches, they have just the caudal and cranial physes. Based on the single longitudinal sections of this rat’s tail, the defects are consistent with a segmented hemivertebra and a butterfly vertebra; however, two view radiographs, MRI, or serial sections would be needed to confirm the diagnosis.
Valproic acid is a short chain fatty acid used to treat epilepsy, migraine, and bipolar disorder. Actions of the drug include modulation of neurotransmission and epigenetic regulation of gene expression. In humans, prenatal exposure to valproic acid has been associated with an increased risk of autism spectrum disorders, as well as a variety of congenital malformations.9 Rodents exposed to valproic acid in utero are used as an animal model of autism.6
Although the exact pathogenesis is not known, proposed mechanisms of action for valproic acid-induced malformations include intracellular acidification, changes in lipid, zinc, folate, or retinoid metabolism, and alterations in developmental gene expression.9
Contributing Institution:
Virginia-Maryland College of Veterinary Medicine
Blacksburg, VA
https://vetmed.vt.edu/
JPC Diagnosis:
Tail: Vertebral malformation, multisegmental, with hemi- and butterfly vertebra formation.
JPC Comment:
As the contributor notes, the development of the vertebral column is complex, with the vertebral bodies forming from proliferating and migrating sclerotomes that surround the embryonic notochord.4 This complex process is orchestrated via spatially and temporally discrete gene expression and cell signaling casacades that shape and position each vertebra in the proper cranial-caudal orientation.4
Even the most casual of anatomist will notice the segmental nature of the vertebrate spine and the different vertebral morphologies displayed at different levels of the spinal column. These patterning differences develop under the influence of Hox genes, which have been extensively studied in mice.7 Researchers have painstakingly mapped the Hox genes to particular functions, finding, for instance, that Hox10 expression deactivates the rib-forming program in non-thoracic ribs and, when expression is experimentally blocked, lumbar vertebrae grow ribs.7 In snakes, Hox10 genes have lost the ability to block rib formation, leading to rib growth down the entire length of the animal.7
Sonic hedgehog (Hh) signaling is another critical player in embryonic patterning and development. While Hh signaling is broadly important in the development of the face, skull, limbs, and axial skeleton, in the vertebral column, Hh signaling from the notochord is crucial in initial somite formation and the development and maintenance of intervertebral discs in development; in post-natal life, when the notochord becomes the nucleus pulposus, Hh signaling is key for the maintenance of intervertebral disc integrity.4,10
These two patterning pathways are just two among many, and any missteps in this complex developmental dance produce congenital defects in the formed vertebra. This case nicely demonstrates, and the contributor nicely describes, two such morphologic defects, but there are many others. In humans, aberrant expression of Hox and/or Hh signalling have been associated with a syndrome clunkily named VACTERL, for vertebral anomalies (V), anal atresia (A), cardiac malformation (C), tracheoesophageal fistula (TE), renal dysplasia (R), and limb abnormalities (L).4 This clinical syndrome is diagnosed when a patient has three or more of the listed abnormalities, and among the listed defects, vertebral abnormalities are found in up to 95% of patients.4 Perhaps a more familiar consequence of vertebral developmental defects is spina bifida, a congenital anomaly in which the vertebral arch fails to fuse. While the underlying pathogeneses have not been fully worked out, there is mounting evidence that abnormal Hox gene expression is the root cause of a number of neural tube defects, including anencephaly, spina bifida, hydrocephaly, and encephalocele.12
The complications of developmental anatomy were well illustrated on the histologic slide examined in conference. The moderator began by noting that the first location clue in this challenging slide is the prominent nucleus pulposus surrounded by the concentric rings of the annulus fibrosis. These features definitively localize the tissue to the vertebral column. In a rat, the nucleus pulposus should be a discoid shape and the affected vertebral joints in section feature very distorted architecture, indicating that the lesion is architectural and, therefore, likely developmental. The moderator agreed with the contributor that the exact nature of the vertebral malformations would be best assessed via advanced imaging, but that hemivertebra and butterfly butterfly formation were most likely based on the histologic features.
A common thread in this week’s conference discussion was the perceived difficulty of evaluating bone pathology. In her closing thoughts, the moderator challenged that assertion with the view that bone responds to injury in a stereotypic manner. The key to understanding bone pathology, then, is understanding how bone behaves normally; once one understands how bone develops and how it undergoes modeling and remodling in response to injury, then the pathology that you see can be essentially reverse engineered to narrow down the universe of potential pathologic insults.
Discussion of the morphologic diagnosis was mercifully straightforward with this case. Participants preferred to call the distribution multisegmental to emphasize that multiple vertebrae were affected, but the contributor’s diagnosis was otherwise adopted largely intact.
References:
- Burbidge HM, Thompson KC, Hodge H. Post natal development of canine caudal cervical vertebrae. Res Vet Sci. 1995;59 (1):35-40.
- Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development, and function. Cell Tissue Res. 2017;370(1):53-70.
- Gutierrez-Quintana R, Guevar J, Stalin C, Faller K, Yeamans C, Penderis J. A proposed radiographic classification scheme for congenital thoracic vertebral malformations in brachycephalic “screw-tailed” dog breeds. Vet Radiol Ultrasound. 2014; 55(6):585-591.
- Kalamchi L, Valle C. Embryology, Vertebral Column Development. StatPearls Publishing; 2024. Available at https://www.ncbi.nlm.nih.gov/books/NBK549917.
- Katsuura Y, Kim HJ. Butterfly vertebrae: a systematic review of the literature and analysis. Glob Spine J. 2019;9(6):666-679.
- Mabunga DFN, Gonzales ELT, Kim J, Kim KC, Shin CY. Exploring the validity of valproic acid animal model of autism. Exp Neurobiol. 2015;24(4):285-300.
- Mallo M, Wellik DM, Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev Biol 2010;344(1): 7-15.
- Moon SM, Yoder JH, Wright AC, Smith LJ, Vresilovic EJ, Elliott DM. Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. Eur Spine J. 2013;22(8):1820-1828.
- Padmanabhan R, Abdulrazzaq YM, Bastaki SMA. Valproic acid-induced congenital malformations: Clinical and experimental observations. Congenit Anom. 2000;40(4):259-268.
- Rajesh D, Dahia CL. Role of sonic hedgehog signaling pathway in intervertebral disc formation and maintenance. Curr Mol Biol Rep. 2018;4(4):173-179.
- Wang Y, Battié MC, Videman T. A morphological study of lumbar vertebral endplates: radiographic, visual and digital measurements. Eur Spine J. 2012;21(11): 2316-2323.
- Yu J, Wang L, Pei P, et al. Reduced H2K27me3 leads to abrnomal Hox gene expression in neural tube defects. Epigenetics Chromatin. 2019;12:76.