WSC 2023-2024, Conference 20, Case 2
Signalment:
Three month old female Quarter Horse (Equus caballus)
History:
A 3-month-old Quarter Horse filly presented to the Iowa State University Equine Internal Medicine Service for diarrhea of two weeks duration. The foal had been treated with omeprazole and sucralfate and several courses of trimethoprim sulfa and ceftiofur with no resolution of clinical signs.
Gross Pathology:
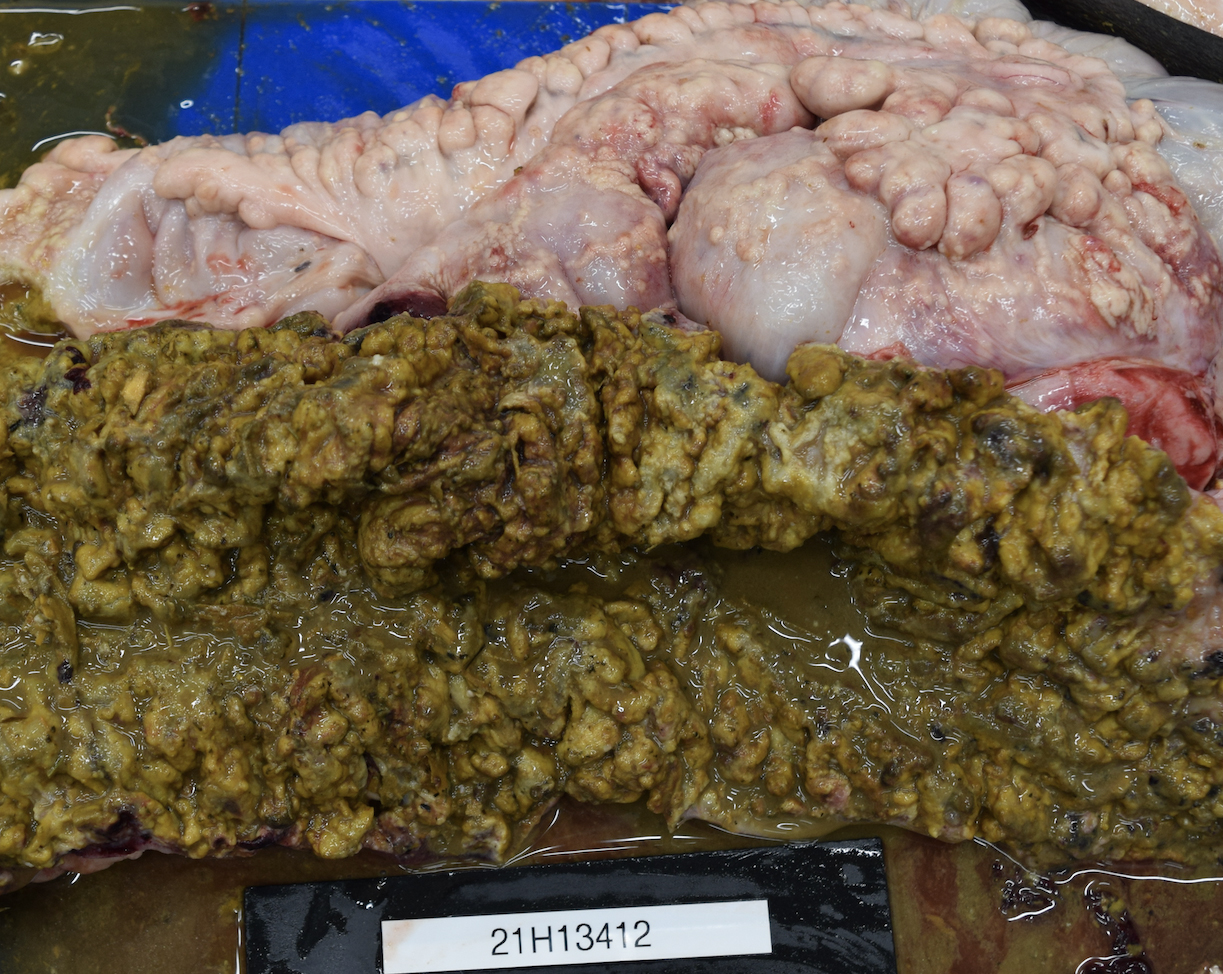
There were severe multifocal to coalescing, 0.1 cm to 5 cm pale nodules in all lung lobes. Some nodules were cream-colored with a friable consistency and were surrounded by a firm, white capsule. Others had a pale yellow liquid exudate exuding from the center of the nodule. The jejunum had multifocal, up to 0.5 cm in diameter ulcerations. Throughout the large colon and cecum, there were multifocal to coalescing ulcerations of the mucosa, which were covered by thick, yellow to dark green, friable material. On the serosal surface, mostly along the mesentery, there were numerous multifocal to coalescing nodules containing caseous yellow exudate. These nodules had a thick white capsule and ranged in size from 1 mm to 5 cm. The peritoneal cavity contained yellow, cloudy fluid.
Laboratory Results:
A fecal sample was submitted to UC Davis for foal gastrointestinal and diarrhea panel testing (Clostridium difficile toxins A and B, Equine coronavirus, Lawsonia intracellularis, Salmonella spp, Cryptosporidium spp, equine rotavirus, Rhodococcus equi, Clostridium perfringens antigen and toxins CPA, CPB, CPB2, netF, and CPE). The sample was positive for Rhodococcus equi and Rhodococcus VapA gene was confirmed by PCR.
Microscopic Description:
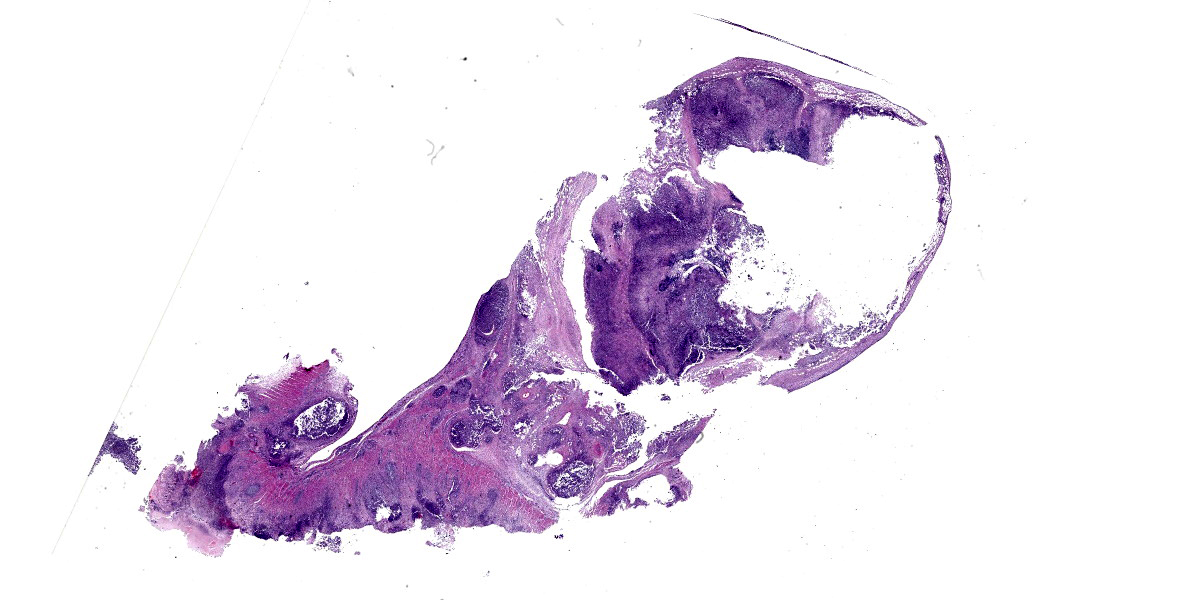
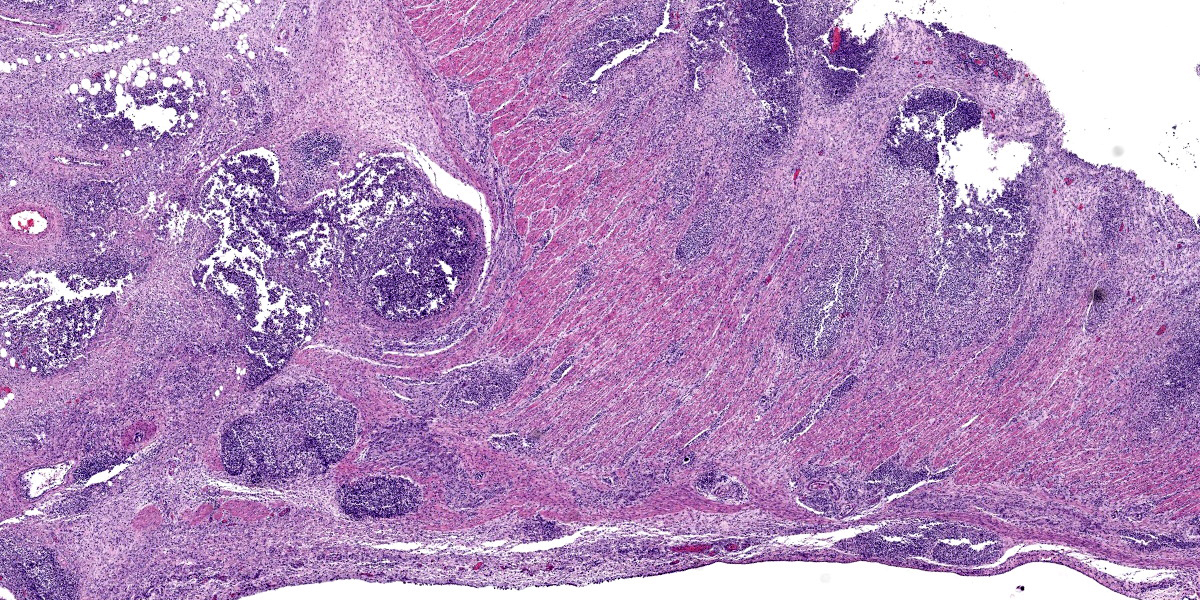
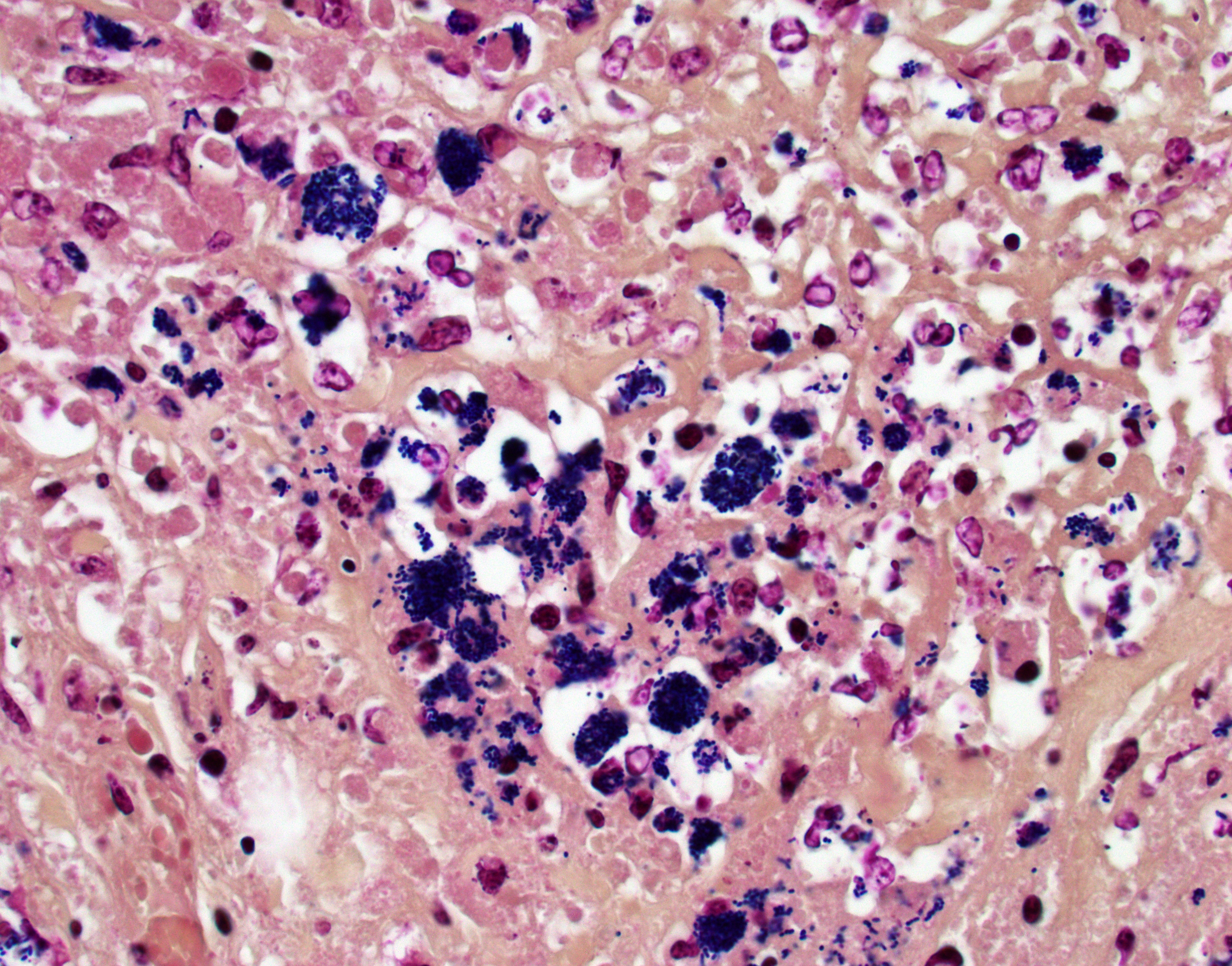
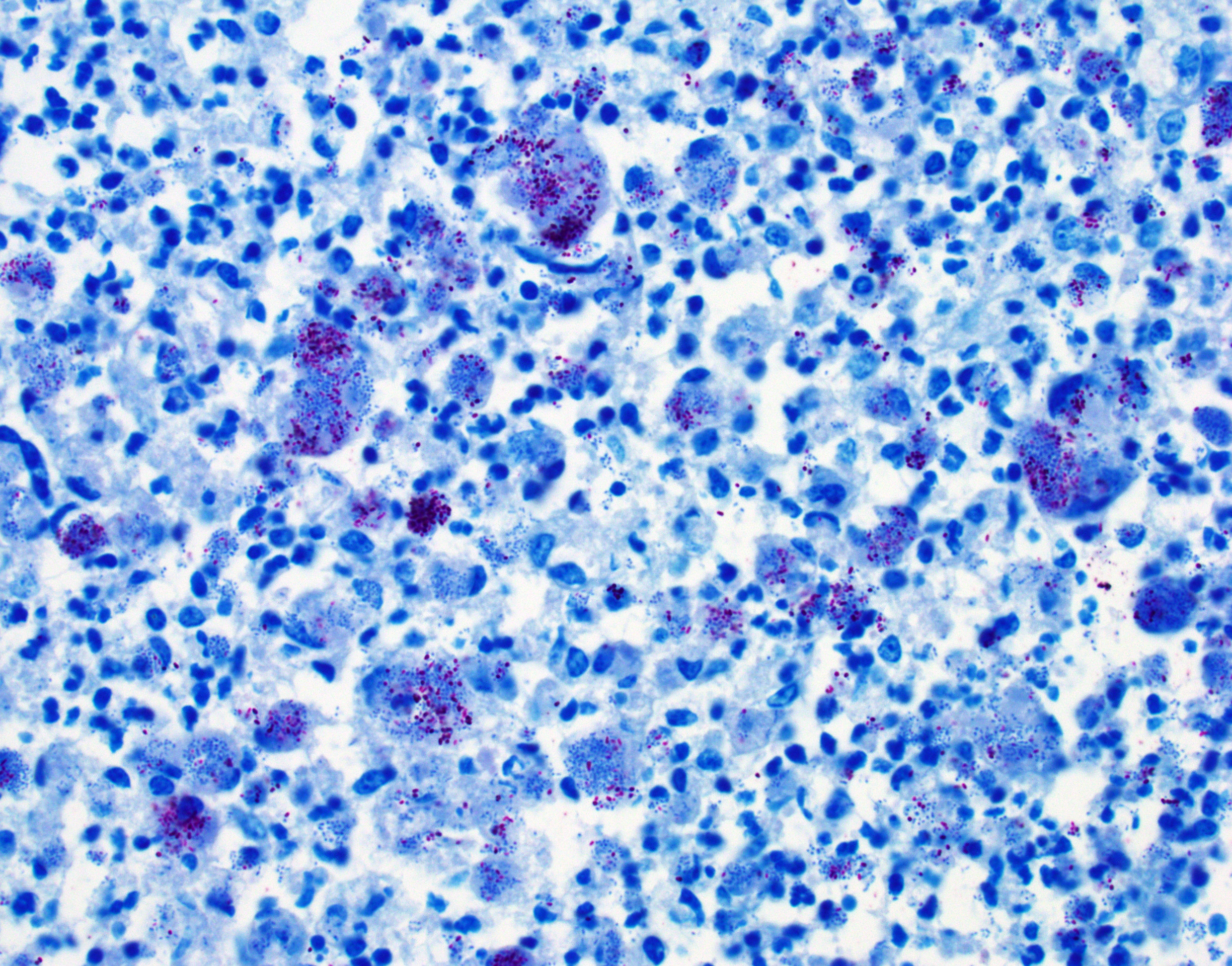
Colon, cecum, and mesenteric lymph node: Elevating the ulcerated mucosa, expanding the submucosa and muscularis with extension to the serosa and the nearby lymph node are multifocal to coalescing aggregates of hypereosinophilic coagulum surrounded by numerous degenerate neutrophils admixed with plump foamy macrophages, epithelioid macrophages and multinucleated giant cells that frequently contain clusters of basophilic 1 to 3 um coccobacilli (pyogranulomatous inflammation with liquefactive necrosis). The mucosa is partially or completely devoid of epithelium and is replaced by fibrinonecrotic debris and similar inflammatory cells surrounded by proliferated fibrous connective tissue. Multifocally, blood vessels are occluded by numerous degenerate inflammatory cells, foamy macrophages with intracytoplasmic coccobacilli, and hyalinized fibrinous meshes. Within the mesenteric lymph node, there is a complete loss of nodal architecture and the cortex and medulla are replaced by pyogranulomas.
Histochemical staining: The intrahistiocytic coccobacilli are highlighted by Gram stain (in blue color) and Fite's stain.
Contributor’s Morphologic Diagnoses:
- Colon and cecum: Severe, multifocal to coalescing, chronic, pyogranulomatous, fibrinonecrotic typhlocolitis, with numerous intrahistiocytic coccobacilli.
- Lymph nodes: Severe, multifocal to coalescing, chronic, pyogranulomatous lymphadenitis with numerous intrahistiocytic coccobacilli.
Contributor’s Comment:
Rhodococcus equi is a gram-positive, facultative intracellular bacterium commonly found in soil and the gastrointestinal tract of herbivores.6 The pathogen primarily causes diseases in foals and also affects cattle, sheep and goats, pigs, dogs, llamas, and immunocompromised humans.1,3,6 Infections of Rhodococcus equi usually occur in foals between 1 and 6 months of age and usually present as pyogranulomatous bronchopneumonia and ulcerative enterocolitis.6
In horses, inhalation of the bacterium from the environment and swallowing of mucus-containing Rhodococcus equi from the airway are the major routes of infection.3,6 R. equi is phagocytosed by macrophages and dendritic cells through the mannose-binding receptor, and the uptake of the bacteria is enhanced by complement and complement receptor 3.9 With plasmid pathogenicity island (PAI)-encoded virulence-associated protein A (VapA) gene and positive regulators (orf4 and orf8), Rhodococcus equi can survive and replicate in macrophage phagosomes through inhibition of phagosome acidification and phagosome-lysosome fusion.6,7,9 Hematogenous dissemination within macrophages to other sites in the body then occurs. Horizontally-acquired chromosomal virulence factors, such as a polysaccharide capsule, mycolic acid, lecithinase, phospholipase C, and cholesterol oxidase also play key roles in the development of systemic pyogranulomatous inflammation.6,7,9 In goats and pigs, R. equi with VapN and VapB plasmids, respectively, are considered the virulent strains that cause systemic pyogranulomatous inflammation.7 R. equi without the aforementioned Vap genes are classified as avirulent.4
In foals, multifocal to coalescing nodules with caseous centers usually develop in the cranioventral lung, but all lung lobes may be affected in severe cases.6 Volcano ulcers covered by necrotic debris are seen over Peyer’s patches in the small intestine, cecum, and large colon.6 The nearby lymph nodes are enlarged with variably-sized abscesses. The histologic features of R. equi infection include suppurative to pyogranulomatous bronchopneumonia with numerous neutrophils, macrophages, and multinucleated giant cells. In the intestine, infiltration of macrophages and neutrophils in the lymphoid follicles and caseous necrosis that extends to the covering epithelium are characteristic features. With time, the mucosa is covered with thick fibrinonecrotic debris. Some foals can also develop polyarthritis, osteomyelitis, and abscessation in multiple internal organs.6,7 Infrequently, abortion caused by R. equi has been described in horses, with fetal lesions comparable to those in foals.5 With special histochemical stains, R. equi is gram-positive and weakly acid-fast.
Differential diagnoses for systemic pyogranulomatous inflammation in horses include fungal and mycobacterial infections; however, concurrent pyogranulomatous pneumonia and ulcerative enterotyphlocolitis are characteristic of R. equi. Bacterial culture with positive detection of the VapA gene in isolated R. equi can confirm the diagnosis.
Contributing Institution:
Department of Veterinary Pathology
College of Veterinary Medicine
Iowa State University
https://vetmed.iastate.edu/vpath
JPC Diagnoses:
- Colon: Colitis, pyogranulomatous and necrotizing, chronic, multifocal to coalescing, severe, with pyogranulomatous lymphangitis and edema, and numerous intrahistiocytic and free coccobacilli.
- Lymph node: Lymphadenitis, pyogranulomatous and necrotizing, chronic, multifocal to coalescing, severe, with numerous intrahistiocytic and free coccobacilli.
JPC Comment:
As the contributor notes, pathogenic Rhodococcus equi strains have the ability to replicate unimpeded within equine macrophage vacuoles.2 The ability to persist inside these vacuoles is dependent on the rapid and abundant production of virulence-associated protein A (VapA), which is triggered primarily by temperatures above 33ºC, as occurs in mammalian hosts, and secondarily by low environmental pH.2 Once expressed, VapA localizes to the bacterial membrane where it interacts with or is released into the vacuolar environment.2,8
VapA promotes the intracellular survival of R. equi by increasing phagosome and lysosome pH. Reserachers were initially surprised to find lysosomal acidification affected by VapA since R. equi multiply, and VapA is produced, within special phagocytic vacuoles that are distinct from host cell lysosomes; however, further research demonstrated that VapA is transferred from the phagocytic vacuole to lysosomes early in infection, possibly by vesicular transport.8 In both the phagocytic vacuole and the lysosome, secreted VapA raises the pH of the compartment, leading to increased proliferation of R. equi and inactivation of acid hydrolases that would normally kill and digest the organisms.2
VapA raises the pH of intracellular compartments via two main mechanisms, the first of which is the exclusion of the proton-pumping ATPase (vATPase) from the phagosome membrane.8 In normal cellular metabolism,
lysosomal and phagosomal membranes are enriched with hundreds of vATPase complexes which acidify the lysosome by pumping hydrogen ions from the cytosol into the lysosome. In phagosomes and lysosome containing VapA, however, the plasma membranes have minimal, background levels of vATPase complexes, and this vATPase exclusion continues with prolonged infection times, preventing rapid acidification of lysosomes.8 The exact mechanism of the vATPase exclusion remains unknown.
VapA also raises the pH of lysosomes and phagosomes by permeabilizing their lipid membranes, further dissipating the acidic proton gradient.2,8 Studies have demonstrated that this permeabilization in vivo is sufficient to allow the release of protons or protonated water, but not complete enough to lead to lysis of the phagosomal or lysosomal membranes.2 The end result of these two mechanisms is to create “leaky,” semipermeable intracytoplasmic niches of relatively neutral pH where R. equi can thrive.
The intracellular thriving of R. equi is well illustrated in this slide, with scores of coccobacilli packed within macrophages and free throughout the examined tissue. The tremendous tissue damage wrought by R. equi once again made tissue identification difficult for conference participants, though most were able to narrow the tissue to either cecum or colon. Some participants remarked on prominent smooth muscle in the wall of many vessels in section; however, the moderator noted that this, along with occasional mineralization, is a common, incidental feature of equine vasculature.
The moderator noted that VapA production is necessary for pathogenesis and that R. equi strains without the plasmid-encoded gene are clinically inert. While an etiologic diagnosis of R. equi infection may technically require PCR for the VapA gene, the moderator noted that R. equi has a distinctive look (pyogranulomas, volcano ulcers) and distribution (lungs and colon) that make the diagnosis fairly straightforward if you have good old-fashioned horse sense. This is particularly true when, as in this case, the tremendous tissue destruction provides strong gross and histologic circumstantial evidence of pathogenicity.
References:
- Bryan LK, Clark SD, Díaz-Delgado J, et al. Rhodococcus equi infections in dogs. Vet Pathol. 2017;54(1):159-163.
- Hansen P, Haubenthal T, Reiter C, et al. Differential effects of Rhodococcus equi virulence-associated proteins on macrophages and artificial lipid membranes. Microbiol Spect. 2023;11(2):e0341722.
- Löhr CV, O'Neill TW, Daw DN, Pitel MO, Schlipf JW. Pyogranulomatous enteritis and mesenteric lymphadenitis in an adult llama caused by Rhodococcus equi carrying virulence-associated protein A gene. J Vet Diagn Invest. 2019;31(5):747-751.
- Stranahan LW, Plumlee QD, Lawhon SD, Cohen ND, Bryan LK. Rhodococcus equi infections in goats: characterization of virulence plasmids. Vet Pathol. 2017;55(2): 273-276.
- Szeredi L, Molnár T, Glávits R, et al. Two cases of equine abortion caused by Rhodococcus equi. Vet Pathol. 2006;43(2):208-211.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Elsevier; 2016:197-198.
- Vázquez-Boland JA, Giguère S, Hapeshi A, MacArthur I, Anastasi E, Valero-Rello A. Rhodococcus equi: the many facets of a pathogenic actinomycete. Vet Microbiol. 2013;167(1-2):9-33.
- von Bargen K, Scraba M, Kramer I, et al. Virulence-associated protein A from Rhodococcus equi is an intercompartmental pH-neutralising virulence factor. Cell Microbiol. 2019;21(1):e12958.
- Zachary JF. Mechanisms of microbial infections. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsevier Mosby; 2017:168-169.