Signalment:
Gross Description:
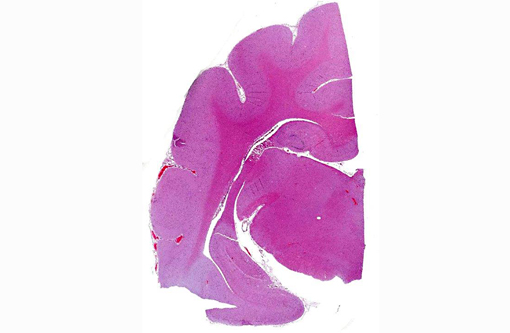
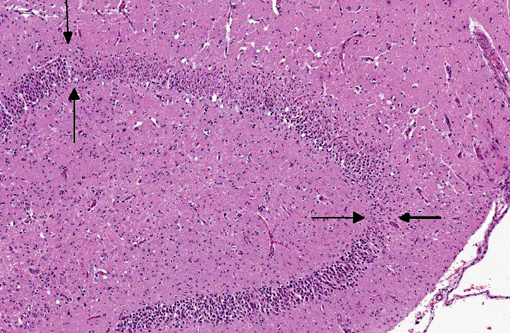
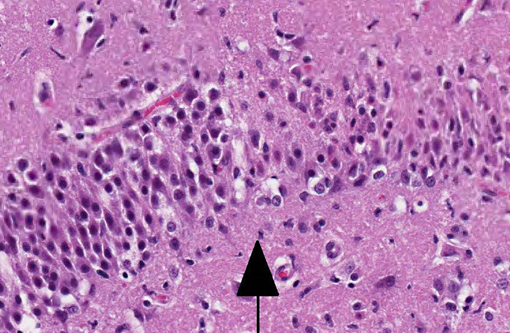
Histopathologic Description:
Other relevant histological findings: In the lungs there was severe congestion and edema. Pulmonary vessels were normal. There was moderate acute congestion in the liver. Although renal cortical petechiae were detected macroscopically, this could not be confirmed histologically, but the glomerular capillaries were engorged with blood. Cell density of bone marrow was moderately increased with mixed cell types but dominated by erythroid cells of mature stages.
Morphologic Diagnosis:
Lab Results:
| Complete blood count: | |
| RBC: 20.56 (5-10) | |
| HGB: 239 (80-150) | |
| HCT: 0.72 (0.24-0.45) | |
| MCV: 35.1 (40-52) | |
| RDW: 18.5 (13-17) | |
| Lymphocytes: 0.2 (1.5-7.0) | |
| Conclusion: marked erythrocytosis and marked lymphopenia | |
Condition:
Contributor Comment:
The polycythemia observed in this cat was interpreted to be an absolute polycythemia (primary or secondary) and not a relative polycythemia due to dehydration, since the cat presented with a high hematocrit (0.72) even after rehydration. The rare disease primary polycythemia (polycythemia vera) must be distinguished from secondary polycythemia which is more common. In primary polycythemia, the bone marrow has a very high cellularity with little remaining fat. Aspirated marrow has a synchronous trilineage hyperplasia with a myeloid:erythroid ratio of near 1.0.(9) This cat had had a moderately cellular bone marrow with a dominance of mature erythroid cells, possibly indicating a secondary polycythemia. Measurement of blood oxygen levels would be helpful differentiating between primary and secondary polycythemia, but this was not performed in this case. A cause of secondary polycythemia is vascular anomalies causing anoxia. This cat had a persistent foramen ovale of 8 mm in diameter, a lesion usually not causing anoxia unless the blood flow through the atrial defect switches from left-right to right-left (Eisenmenger syndrome).(5) Pulmonary hypertension may be the result of heart malformations causing left to right shunting of blood and increased blood flow to the lungs.(1) Vascular lesions in the lungs due to pulmonary hypertension were not detected in this cat; neither acute lesions consisting of endothelial degeneration, fibrinoid necrosis and vasculitis, nor chronic lesions consisting of remodeling of pulmonary arterioles with thickening of the tunica intima and hypertrophy of the media were noted.(1) Interestingly, hypoxia due to patent foramen ovale in the absence of pulmonary hypertension is described in humans.(4) It is not clear to what degree the cardiac and hematological abnormalities contributed to the hippocampal necrosis in this cat. Of the 38 cats described by Fatzer et al, no similar hematological abnormalities were described; however, it is unclear whether hematological examinations were performed on all cats.
JPC Diagnosis:
Conference Comment:
Due to its use of glutamate (or possibly aspartate) as an excitatory neurotransmitter, the hippocampus is also particularly vulnerable to neuronal excitotoxicity, which may have played a role in the pathogenesis of this case. Glutamate is toxic to neurons and is normally cleared rapidly by glial cells. Conditions such as ischemia, hypoxia, seizures, and hypoglycemia promote a cascade of unregulated events including endogenous glutamate release, activation of glutamate receptors, decreased clearance of glutamate, and opening of voltage gated calcium channels, ultimately resulting in neuronal degeneration and necrosis; this is known as endogenous neuronal excitotoxicity.(6,8) A comparable neurotoxic condition has been described in marine mammals and seabirds in association with exposure to domoic acid (an analog of L-glutamate) following harmful algal blooms.(8)
In contrast to the microscopic lesions in this case, which are confined to the hippocampus, the lymphoplasmacytic perivascular cuffing, focal gliosis and neuronal intracytoplasmic viral inclusions (Negri bodies) commonly associated with rabies virus tend to be most severe from the pons to the hypothalamus and within the cervical spinal cord.(6) Nevertheless, definitive diagnosis of feline hippocampal necrosis in this case (versus rabies viral infection) was complicated for some participants by identification of scattered eosinophilic, intracytoplasmic rabies-like inclusions. The occurrence of intracytoplasmic neuronal inclusions indistinguishable from Negri bodies is a fairly common incidental finding in older dogs and cats which must be differentiated from true viral inclusions with laboratory diagnostics such as fluorescent antibody testing.(7)
References:
1. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Elsevier Limited; 2007:523-654.
2. Coradini M, Johnstone I, Filippich LJ, Armit S. Suspected fibrocartilaginous embolism in a cat. Aust Vet J. 2005;83:550-551.
3. Fatzer R, Gandini G, Jaggy A, Doherr M, Vandevelde M. Necrosis of hippocampus and piriform lobe in 38 domestic cats with seizures: a retrospective study on clinical and pathologic findings. J Vet Intern Med. 2000;14:100-104.
4. Maraj R, Ahmed O, Fraifeld M, Jacobs LE, Yazdanfar S, Kotler MN. Hypoxia due to patent foramen ovale in the absence of pulmonary hypertension. Tex Heart Inst J. 1999;26:306-308.
5. Maxie MG, Robinson WF. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 3. 5th ed. Philadelphia, PA: Elsevier Limited; 2007:1-106.
6. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 1. 5th ed. Philadelphia, PA: Elsevier Limited; 2007:284-285, 336-337, 413-416.
7. Nietfeld JC, Rakich PM, Tyler DE, Bauer RW. Rabies-like inclusions in dogs. J Vet Diagn Invest. 1989;1(4):333-338.
8. Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, Gulland FM. Pathology of domoic acid toxicity in California sea lions (Zalophus californianus). Vet Pathol. 2005;42(2):184-191.
9. Valli VEO. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 3. 5th ed. Philadelphia, PA: Elsevier Limited; 2007:107-324.
10. Vandevelde M, Higgins RJ, Oevermann A. Metabolic-toxic diseases. In: Veterinary Neuropathology: Essentials of Theory and Practice. Chichester, UK: Wiley-Blackwell; 2012:106-128.