Signalment:
Nine-month-old Red Angus steer, (
Bos taurus).This steer belonged to a herd of 250 animals that were weaned one month prior to presentation. This steer had a brief history of inability to stand up and was treated with 6.5 cc Draxxin-� (tulathromycin). Ten other animals in this herd were treated for respiratory disease and twenty were seen limping in the previous week. Another steer in this herd had an enlarged stifle that was tapped and cytology revealed inflammatory arthropathy.
Gross Description:
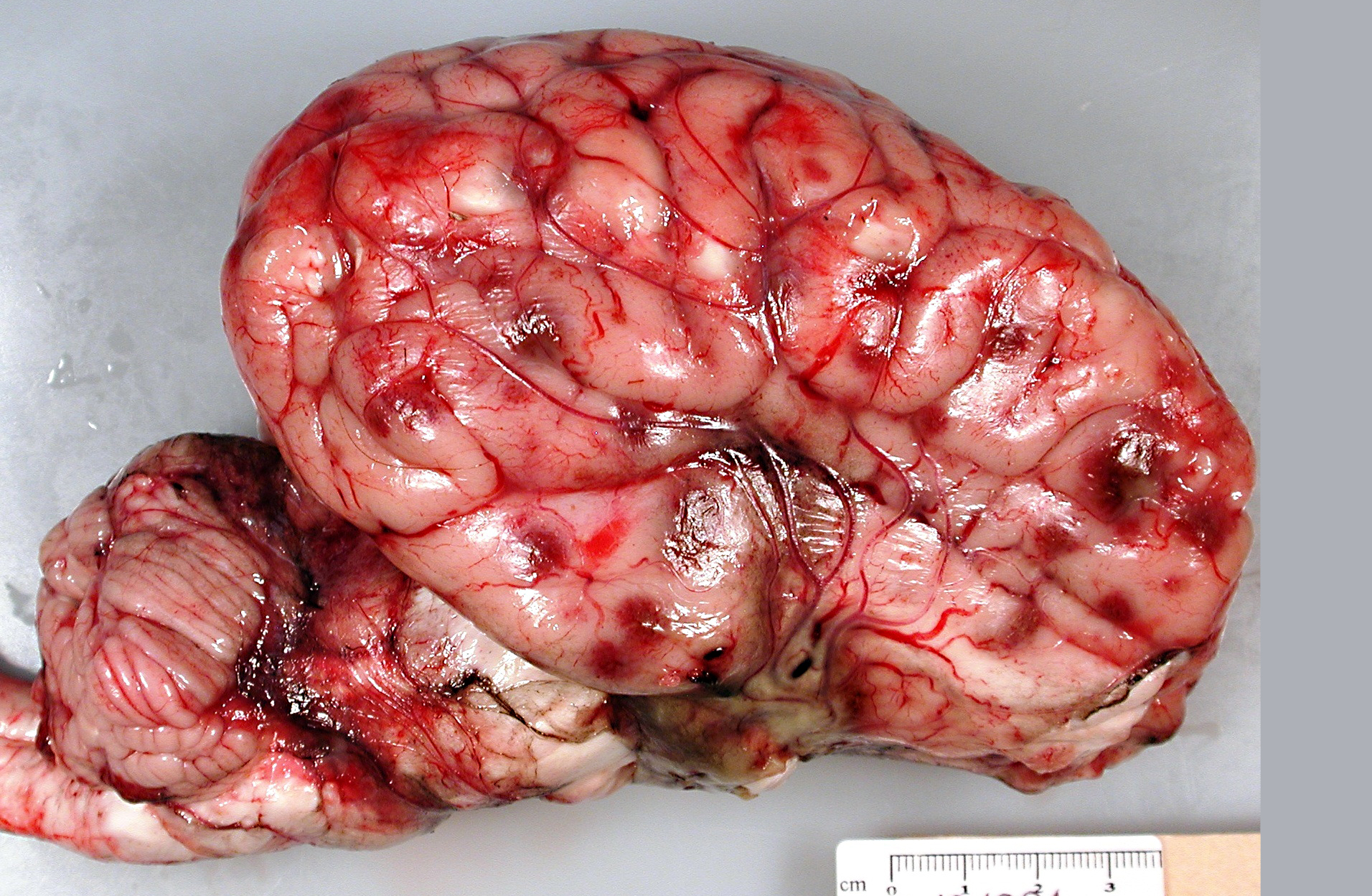
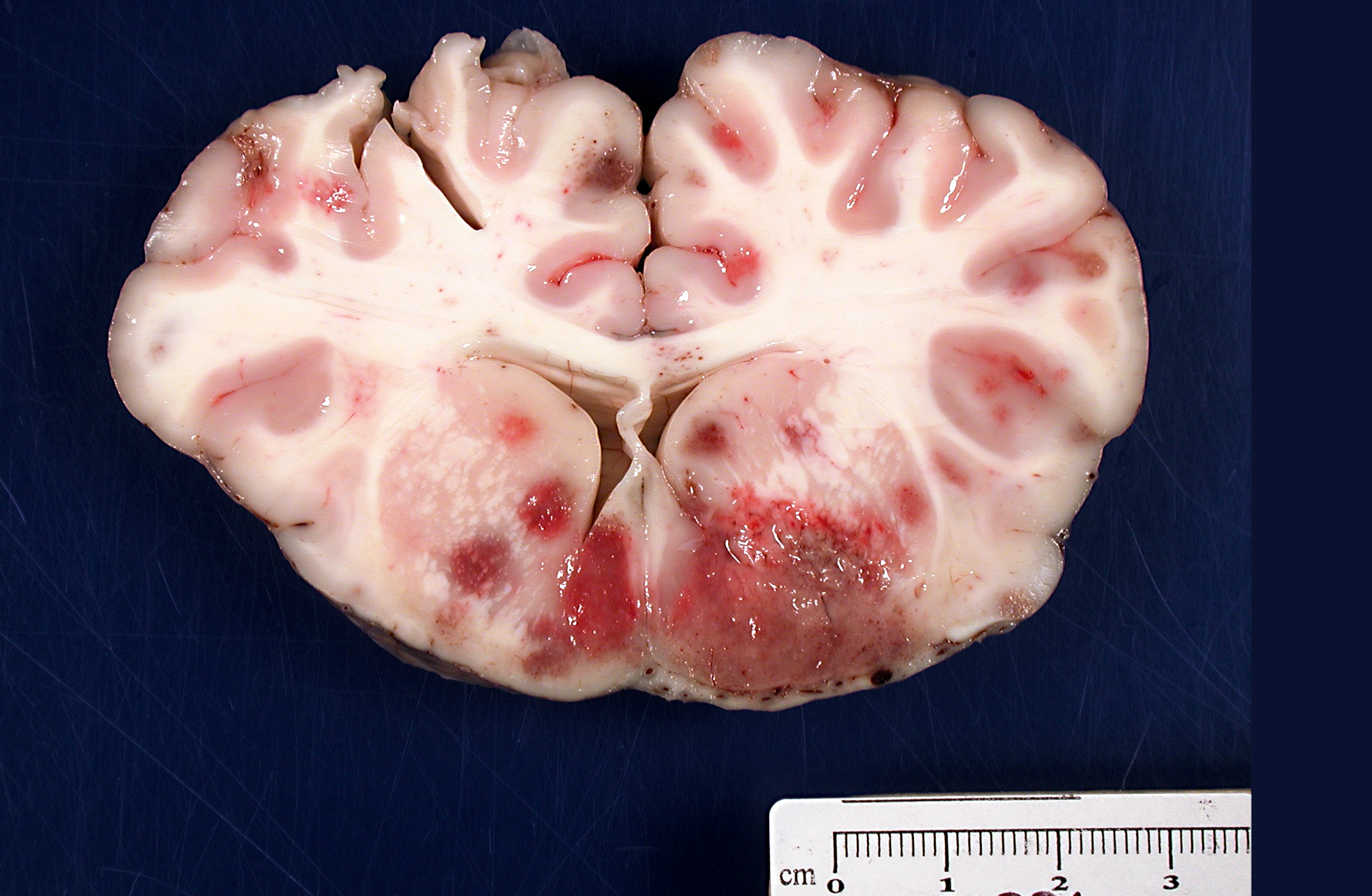
Brain: There were multifocal random foci of malacia and hemorrhage in the gray and white matter of the cerebrum, cerebellum, and brainstem. The meninges in the ventral aspect of the brain contained a moderate accumulation of fibrinosuppurative material. The thoracic cavity contained approximately 50 mL of serous fluid with fibrin strands. The lungs failed to fully collapse upon opening the thoracic cavity. The right cranial and middle lobes and the left cranial lobe were consolidated and attached to the thoracic wall by a thick mat of fibrin (approximately 50% of the total lung). On cut sections of these lobes, there was exudation of small amounts of whitish suppurative material. Fibrin adhesions were also seen in the caudal portion of the right caudal lobe. The pulmonary parenchyma at this level was grossly normal. Stifle joints in both sides contained increased volume of yellowish and watery synovial fluid with fibrin clots. No gross lesions were found in the heart.
Histopathologic Description:
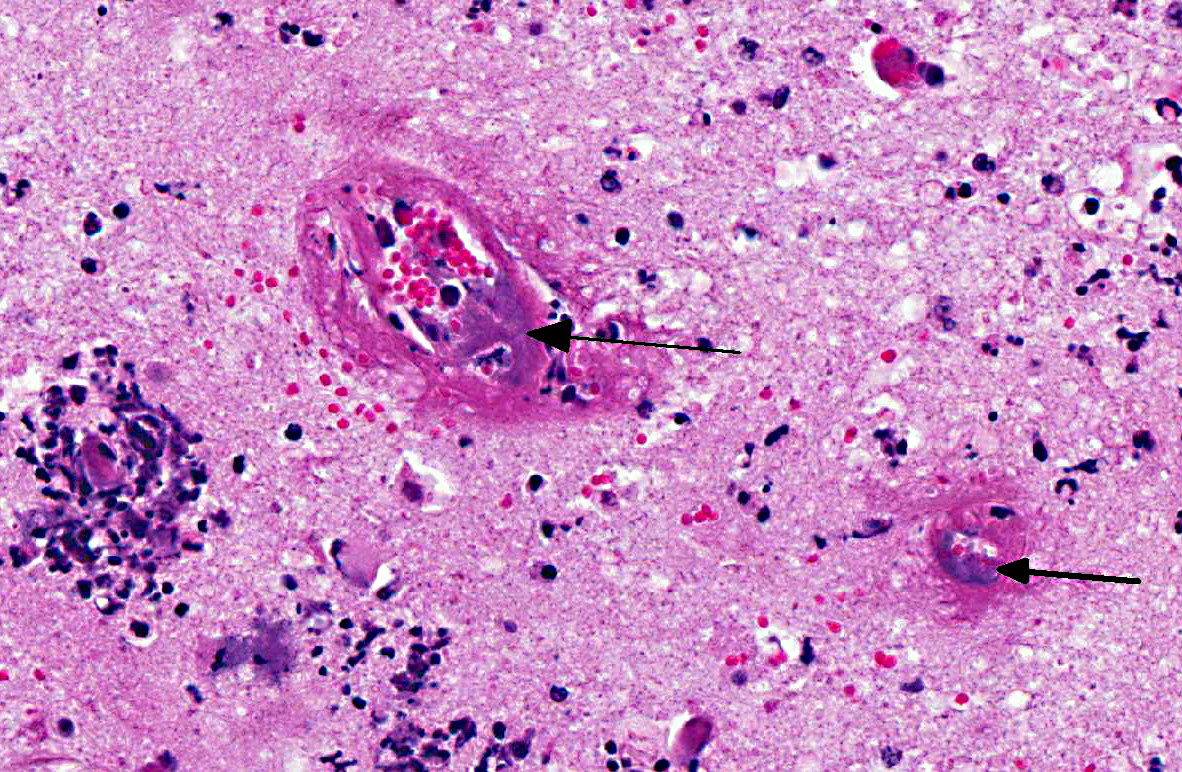
Brain: Sections of cerebrum, cerebellum, or brainstem contain multiple, variably-sized, and random foci infiltrated by moderate to large numbers of neutrophils and lesser numbers of macrophages associated with hemorrhage, edema, degeneration, necrosis and loss of gray and white matter. The meninges over these areas are expanded by sheets of neutrophils and hemorrhage. The tunica media of numerous small to medium sized blood vessels in the meninges and cerebral parenchyma is necrotic and is often effaced by moderate amounts of fibrin (fibrinoid necrosis), and their lumen contains fibrin thrombi. Colonies of Gram negative bacilli are frequently observed within the blood vessels and in surrounding neuropil.Â
Morphologic Diagnosis:
Meningoencephalitis, necrosuppurative and hemorrhagic, multifocal, acute, severe, with necrotizing vasculitis, thrombosis, with intralesional colonies of Gram negative bacilli, etiology consistent with
Histophilus somni.
Lab Results:
Bacterial culture from cerebral swabs and fresh samples of brain yielded growth of
Histophilus somni. Mycoplasma bovis culture from joint swab was positive. Polymerase chain reaction (PCR) for detection of
Mycoplasma bovis, Chlamydia spp., bovine viral diarrhea virus, bovine respiratory syncytial virus, bovine coronavirus, bovine herpesvirus type 1, and parainfluenza type 3 from lung tissue were all negative. Bacterial cultures from the lung yielded no growth of significant pathogens.
Condition:
Histophilus somni
Contributor Comment:
Histophilus somni (previously known as
Haemophilus somnus) is a Gram negative coccobacillus that causes septicemia and a number of clinical signs in cattle, termed altogether as
Histophilus somni disease complex or histophilosis.(6,7,8) The clinical signs in cattle include pneumonia; myocarditis; polyarthritis; mastitis; genital infection; abortion; reproductive failure; and thrombotic meningoencephalitis (TME), the neurological form of the disease.(1,3,6,7) Septicemia caused by
H. somni develops in cattle in many age groups, but it is more prevalent in young growing cattle in feedlots during the winter time.(3,6,8) Other species that are affected include sheep, bison, and bighorn sheep.(1)
The lesions observed in the central nervous system in this steer are compatible with the neurological form of
H. somni disease complex, or TME, which was confirmed by bacterial culture from cerebral swabs and fresh samples of the brain. Historically, TME was the most common disease presentation of the bacterial infection; however, in recent years, the most common presentations are pleuropneumonia and myocarditis.(6,8,9) The gross lesions of TME are random, but most often localized at the cortical gray matter-white matter junction of the cerebral cortex and in the thalamus, where it is presumed that the bacteria can lodge and replicate in the blood vessels due to changes in vessel diameter and flow patterns.(6,7) The lesions consist of multiple variable sized dark red foci of necrosis and hemorrhage.(6,7) The meninges over the hemorrhagic areas are usually affected.(6,7) Similar lesions are also observed in the brainstem and spinal cord.(6,7) Microscopic lesions are characterized by severe vasculitis and thrombosis with infarction, and infiltrates of neutrophils and macrophages in the site.(6,7)
H. somni is a commensal of the respiratory and reproductive tracts.(2) The pathogenesis of the septicemia leading to TME in cattle is unknown, but it is believed that some virulent bacterial strains first colonize the surface of the mucous membranes in the upper respiratory tract and invade the circulatory system.(3,7) The bacteria adhere to the endothelial cells, causing vasculitis, thrombosis, and infarction and continue replicating in the thrombus, triggering an inflammatory response.(7) Several studies have shown that virulent strains of
H. somni produce a multitude of virulence factors aimed at evasion of host defenses for colonization of tissues. Virulence factors in
H. somni include, but are not limited to, lipooligosaccharides (LOS), attachment and induction of apoptosis in bovine endothelial cells, intraphagocytic survival, immunoglobulin Fc binding proteins (IgBPs), biofilm formation, histamine production, and integration of phosphorylcholine (ChoP) into its LOS.(2,9) Disease in TME likely occurs as a result of apoptosis of endothelial cells and host inflammation due in part to the presence of endotoxin and the activation of the coagulation cascade, and the recruitment of polymorphonuclear leukocytes and macrophages to sites of infection.(3,9)
JPC Diagnosis:
Cerebrum: Meningoencephalitis, fibrinosuppurative, multifocal, severe, with vasculitis, fibrinoid necrosis, rarefaction, and intravascular bacterial colonies.Â
Conference Comment:
The hallmark lesion of TME is vasculitis and thrombus formation due to endothelial cell damage and subsequent exposure of the underlying extracellular matrix. Although the mechanism of vascular injury by
H. somni is not fully understood, it is thought to be due to apoptosis caused by direct effects of the bacteria and its lipopolysaccharide (LOS) on endothelial cells.(4,5) However, because there is often endothelial damage in areas devoid of bacterial antigen, studies in more recent years have attempted to elucidate additional mechanisms that may contribute to this vasculitis. These studies have suggested that activated platelets likely play an important role in the vascular damage. Although the primary role of platelets lies in maintaining hemostasis, platelets also contribute to vascular inflammation and injury by expressing chemotactic factors (platelet factor 4, lipooxygenase products, RANTES), cytokines (IL-1β), platelet activating factor, and surface molecules (CD40L, FasL, P-selectin)5 that can interact with leukocytes and endothelial cells.(4,5) Additionally, it has been found that platelets activated by
H. somni induce endothelial apoptosis via caspases 8 and 9, and promote endothelial cell production of reactive oxygen species (ROS), which further enhances apoptosis. Conversely, inhibitors of either caspase 8 or 9, or the disruption of ROS activity, were found to decrease endothelial apoptosis.(5) Interestingly, bovine (and human) endothelial cells appear to be resistant to Fas-mediated apoptosis, and it has been suggested that activated platelets may induce apoptosis of endothelial cells via a novel pathway that utilizes caspases 8 and 9 as well as ROS.(5) These findings contribute to our developing understanding of mechanisms of endothelial cell damage not only in
H. somni infections, but in bacterial sepsis in general as well.Â
References:
1. Ward Alton CS, Weiser GC, Anderson BC, et al.Â
Haemophilus somnus (Histophilus somni) in bighorn sheep.Â
Can J Vet Res. 2006;70:34-42.
2. Corbeil LB.Â
Histophilus somni host-parasite relationships.Â
Anim Health Res Rev. 2008;8:151-160.
3. Fecteau G, George LW. Bacterial meningitis and encephalitis in ruminants.Â
Vet Clin Food Anim. 2004;20:363-377.Â
4. Kuckleburg CJ, McClenahan DJ, Czuprynski CJ. Platelet activation by
Histophilus somni and its LOS induces endothelial cell pro-inflammatory responses and platelet internalization.Â
Shock. 2008;29(2):189-196.
5. Kuckleburg CJ, Tiwari R, Czuprynski CJ. Endothelial cell apoptosis induced by bacteria-activated platelets requires caspase-8 and -9 and generation of reactive oxygen species.Â
Thromb Haemost. 2008;99:363-372.
6. Maxie MG, Youssef S. The Nervous system. In: Maxie MG, ed.Â
Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Edinburg, Scotland: Elsevier; 2007:281-457.Â
7. McGavin MD, Zachary JF.Â
Pathologic Basis of Veterinary Disease. 4th ed. St Louis, MO: Elsevier; 2007.Â
8. Radostis OM, Gay CC, Hinchcliff KW, et al.Â
Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 10th ed. Edinburg, Scotland: Elsevier; 2007.Â
9. Siddaramppa S, Inzana TJ.Â
Haemophilus somnus virulence factors and resistance to host immunity.
Anim Health Res Rev. 2004;5:79-93.