WSC 2023-2024, Conference 21, Case 1
Signalment:
11-year-old, intact male German Shepherd Dog (Canis familiaris)
History:
This patient presented to the Emergency Service for evaluation of a cough of 1-week duration that was unresponsive to Baytril and Torbutrol. His cough became acutely worse, at which time he regurgitated and became dyspneic. Over the next 24 hours the patient became lethargic, weakly ambulatory, and febrile (104ºF). Pertinent previous history included chronic intermittent coughing followed by vomiting/regurgitation. Three-view thoracic radiographs showed a bilateral interstitial to alveolar pattern in the cranioventral lung lobes, marked distention of the thoracic esophagus, and a homogenous oblong soft tissue mass in the cranial mediastinum. An inflammatory leukogram was identified on CBC. While hospitalized he became progressively weaker with marked reduction in pelvic limb reflexes, requiring a sling to walk. Euthanasia was elected.
Gross Pathology:
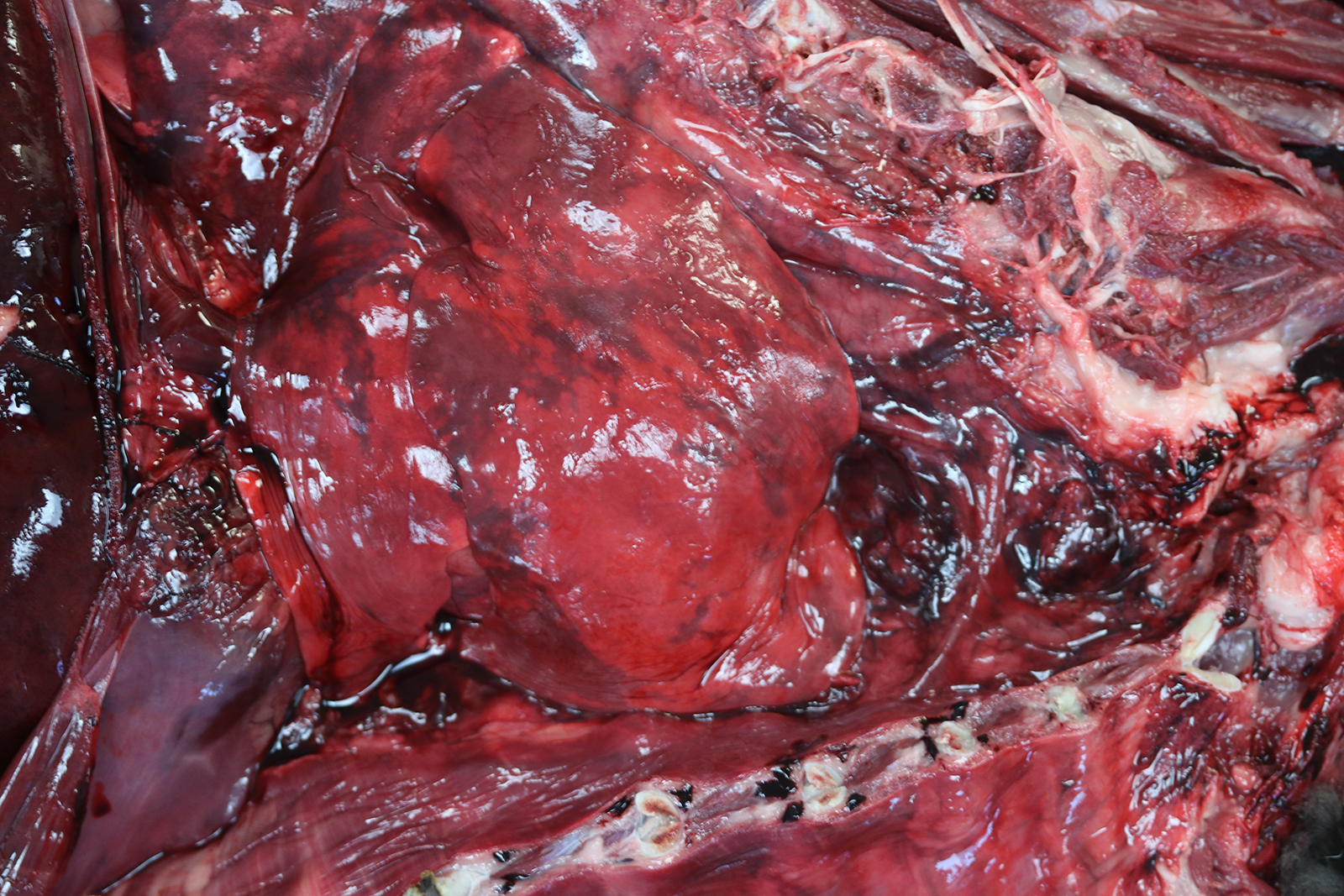
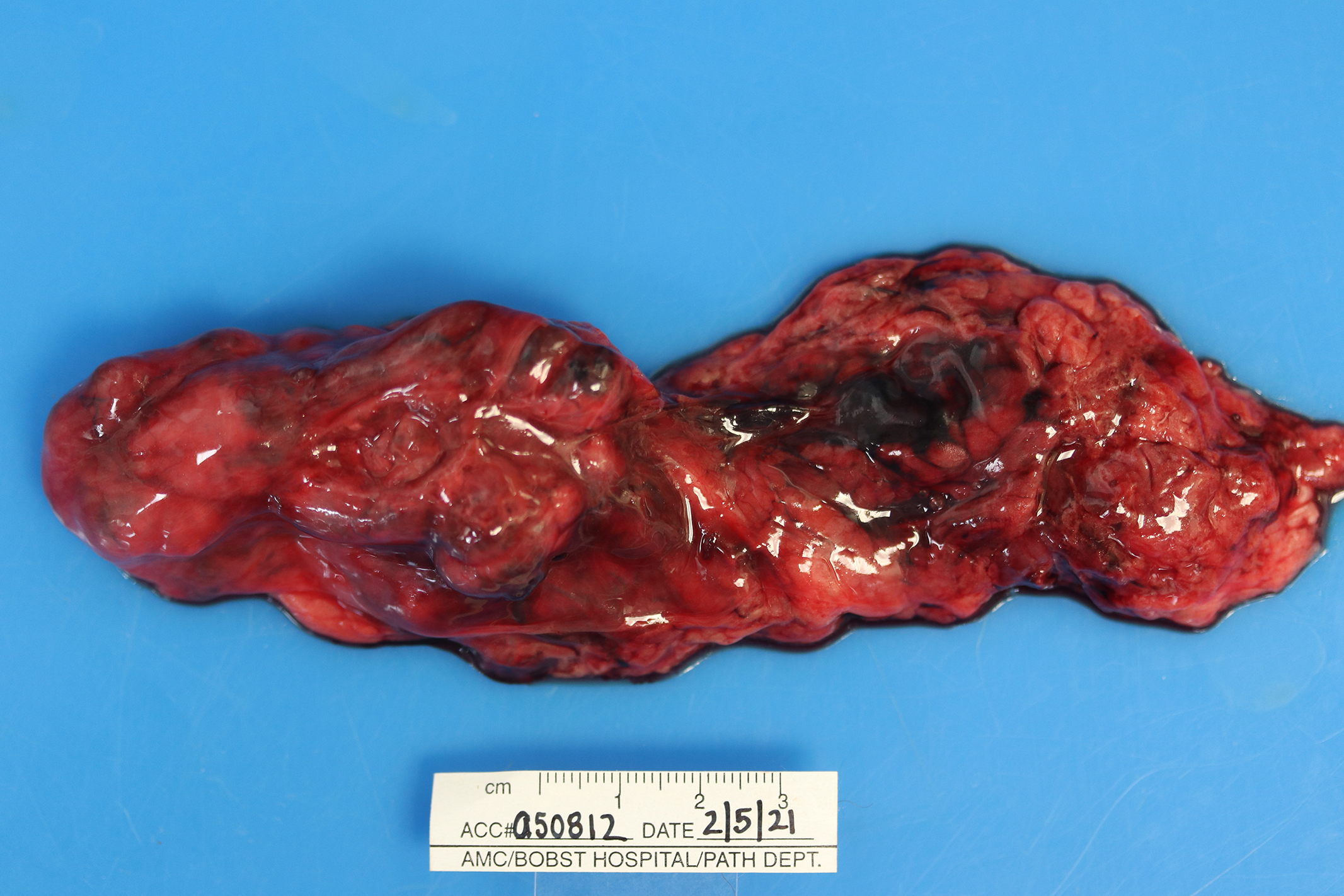
The thoracic cavity contained approximately 2 ml of a non-viscous, translucent, dark red (serosanguinous) fluid. The thoracic esophagus was diffusely distended and was 3.5 cm at its widest dimension. The cranial mediastinum was focally expanded by a poorly demarcated, 14.4 cm x 3.6 cm x 4.5 cm, multilobulated, firm to friable, tan to dark red mass. The cut surfaces of this mass comprised anastomosing, firm, tan trabeculae separated by a soft to friable, dark red tissue.
The right middle lung lobe was diffusely red and firm. Sections from the right middle lung lobe sank in 10% buffered formalin. The right cranial, right caudal, accessory, and left cranial lung lobes were diffusely red and contained multifocal to coalescing, mildly to moderately firm regions. Sections from these lung lobes inconsistently floated, floated low, or sank in 10% buffered formalin. The left caudal lung lobe was diffusely red and rubbery. Sections from the left caudal lung lobe floated in 10% buffered formalin. From the cut surfaces of all lung lobes oozed a moderate amount of non-viscous, translucent, dark red (serosanguinous) fluid.
Laboratory Results:
|
Parameter |
Value |
Reference Range |
|
WBC |
25.8 K/µL |
4.9-17.6 K/µL |
|
Neutrophils |
23.33 K/µL |
2.94-12.67 K/µL |
|
Bands |
258 /µL |
0-170 /µL |
|
Lymphocytes |
0.52 K/µL |
1.06-4.95 K/µL |
|
Monocytes |
1.806 K/µL |
0.13-1.15 K/µL |
|
Eosinophils |
0 K/µL |
0.07-1.49 K/µL |
|
Basophils |
0 K/µL |
0.0-0.1 K/µL |
|
Platelets |
345 K/µL |
143-448 K/µL |
Microscopic Description:
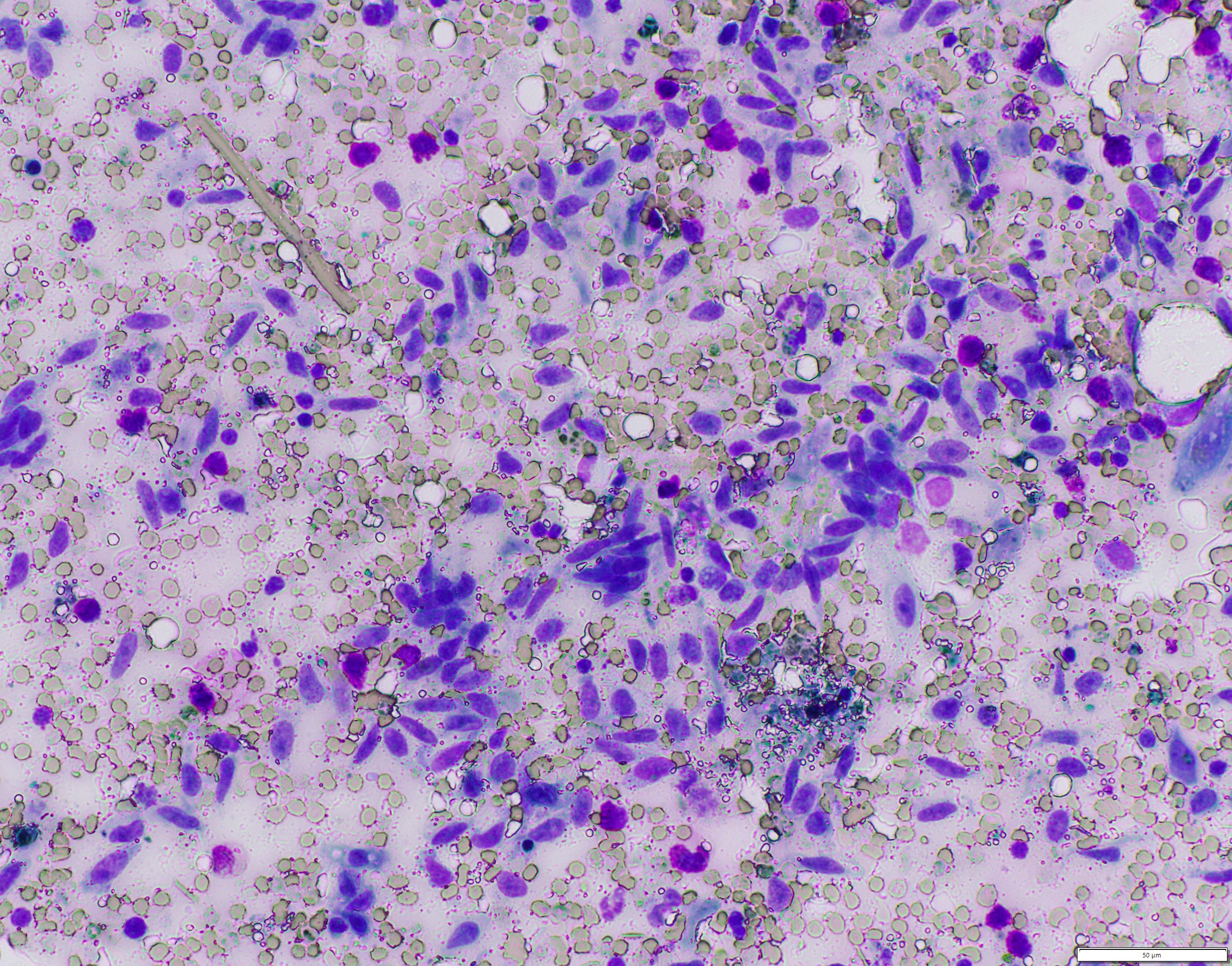
Mediastinal mass cytology: Touch imprints of the cranial mediastinal mass comprise large numbers of streaming, mildly pleocellular spindle cells mixed with small numbers of mast cells, few small to intermediate size lymphocytes, and few macrophages containing variable amounts of hemosiderin on a background of peripheral blood. There are scattered mesothelial cells.
Lung cytology: Touch imprints of the right middle lung lobe comprise abundant degenerate and viable neutrophils and macrophages mixed with small numbers of respiratory epithelial cells on a hemodiluted background with abundant extracellular bacteria. Macrophages infrequently contain hemosiderin. Intracellular bacteria are not identified.
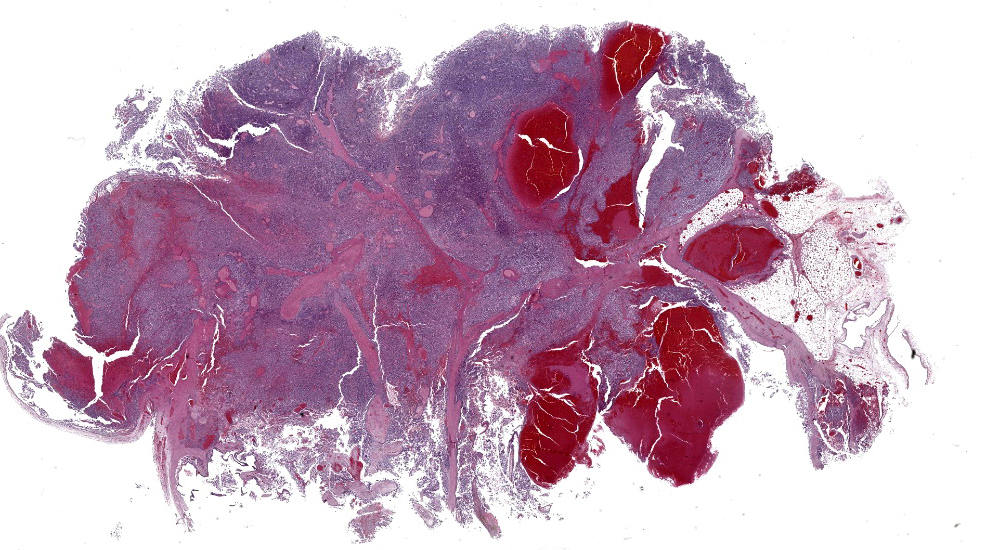
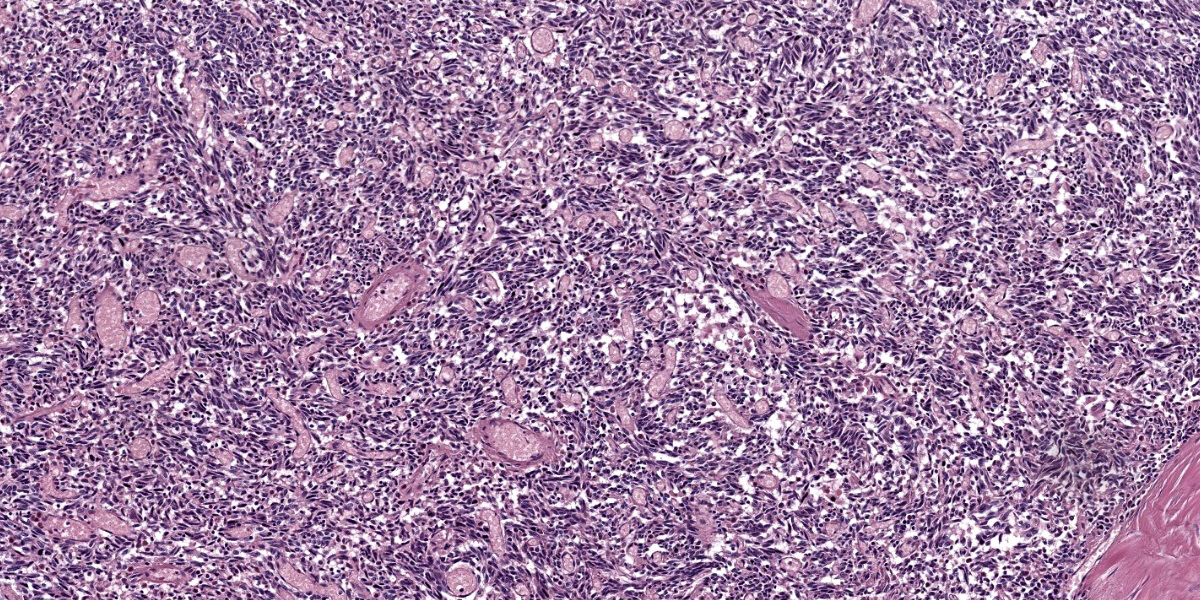
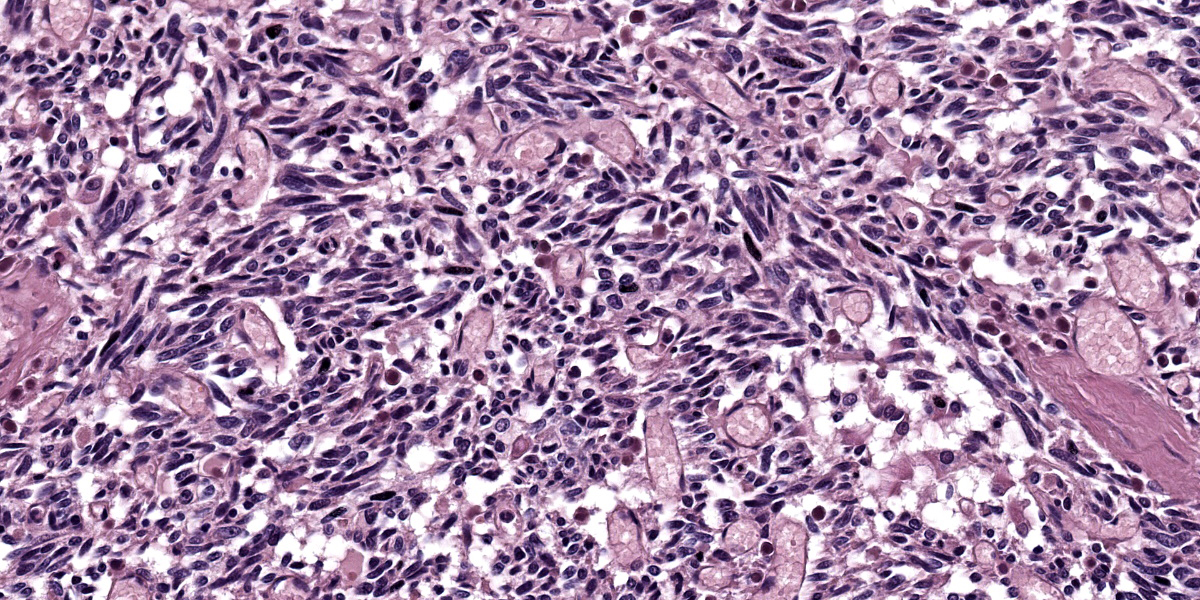
Mediastinal mass histology: Expanding the fibrofatty stroma is a poorly demarcated, unencapsulated neoplasm consisting of mildly pleomorphic oval to spindle to polygonal cells arranged in fascicles, short and long streams, small bundles, and solid regions supported by a fine fibrovascular stroma with congested blood vessels and traversed by dense connective tissue trabeculae. Neoplastic cells are oval to spindle to polygonal with indistinct cell borders and small to moderate amounts of eosinophilic cytoplasm with inconsistent cytoplasmic vacuolization. Nuclei are round to oval with evenly distributed, finely stippled chromatin and up to one prominent nucleolus. No mitotic figures are identified in ten HPF (40x x FN22; 2.37mm2). Scattered within the neoplastic population are a small number of individualized macrophages and small lymphocytes, as well as rare plasma cells. Macrophages infrequently contain intracytoplasmic, deep to golden brown pigment (hemosiderin and lipofuscin). The neoplastic population is multifocally separated by areas of hemorrhage that occasionally form large cavitated regions bordered by neoplastic cells. Rare mineral is present. Hassall’s corpuscles and protein-filled perivascular spaces are not identified.
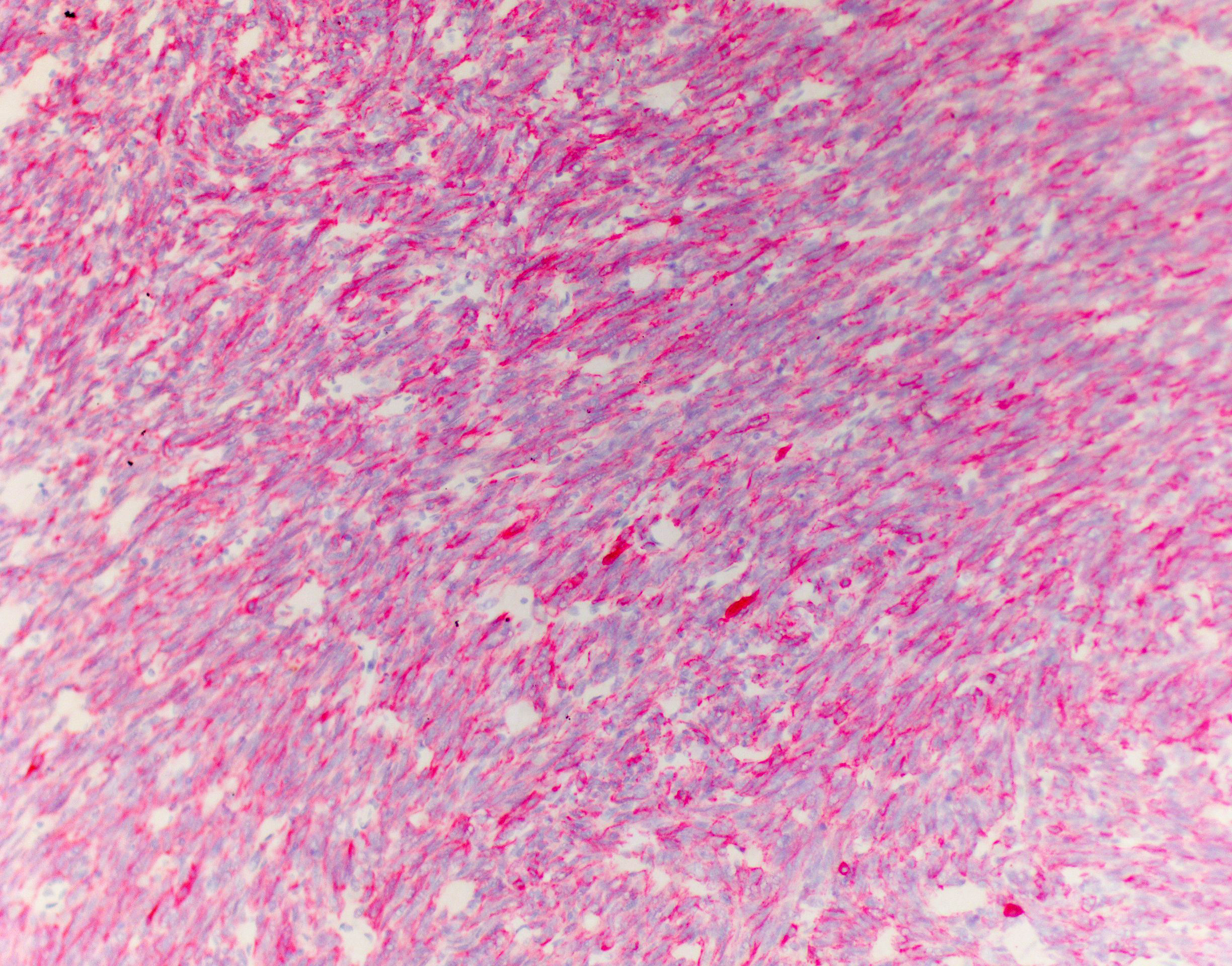
Immunohistochemistry: Approximately 90% of the neoplastic population display moderate to strong, cytoplasmic to membranous immunoreactivity for cytokeratin AE1/3 and are diffusely immunonegative for vimentin. Individualized CD3 immunopositive round cells are scattered throughout the mass and comprise less than 5% of the total cell mass.
Contributor’s Morphologic Diagnosis:
Mediastinal mass: Thymoma, epithelial-rich (prominent spindle cell morphology) with multifocal to coalescing, moderate to severe hemorrhages and few intratumoral small lymphocytes.
Contributor’s Comment:
Based on the clinical presentation, identification of a cranial mediastinal mass with thoracic radiographs, megaesophagus, and an interstitial to alveolar pulmonary pattern in the cranioventral lung lobes, the working clinical diagnosis was thymoma-associated myasthenia gravis with secondary aspiration pneumonia. Gross exam confirmed a multilobular mass in the cranial mediastinum, megaesophagus, pleural effusion, and aspiration pneumonia. Differentials for a cranial mediastinal mass include neoplasia (i.e., thymic epithelial tumor, lymphoma, thymic germ cell tumor, stromal sarcoma, tumors of ectopic thyroid and parathyroid tissues, chemodectoma, lipoma, schwannoma, metastatic disease), thymic cyst, brachial cyst, abscess, and granuloma.14,15,17,23,26 In this case, histopathology supported a diagnosis of an epithelial-rich thymoma with prominent spindle morphology.
The distinction between WHO classified type A and type AB thymomas is difficult, as both share a predominance of bland spindle cells and are distinguished by the percentage of immature T cells (few in type A and high numbers in type AB).9,10 In this case, three representative sections were evaluated as part of the postmortem examination, and all contained a paucity of lymphocytes. These findings would be most consistent with a type A thymoma.
However, as type AB thymomas can have lymphocyte-poor and lymphocyte-rich regions, it is possible that lymphocyte-rich regions were not represented in the examined sections and thus a type AB thymoma could not be entirely ruled out.9,10 As euthanasia was elected before confirmatory AChR antibody titers could be submitted, myasthenia gravis could not be confirmed but was suspected.
As a lymphoepithelial organ, the thymus is formed by a combination of epithelial and lymphoid tissues (differentiating and proliferating T lymphocytes).5,21 In addition, a small population of myoid cells that resemble striated muscle fibers are of unclear origin and thought to play a key role in the development of thymoma-associated myasthenia gravis.21 During development, the thymic epithelium forms from the 3rd pharyngeal pouch’s endoderm that subsequently becomes invaded by blood vessels and connective tissue trabeculae from the adjacent mesoderm and infiltrated by bone marrow derived lymphocyte progenitors.5,8,21 Grossly, the thymus is separated into distinct lobes (paired cervical lobes, an intermediate lobe at the thoracic inlet, and a thoracic lobe), but the arrangement and prominence of each lobe varies among species.5,21 In both ruminants and pigs, the cervical lobes are large.5,6,21 Further, in ruminants the thoracic lobe is in the dorsal part of the cranial mediastinum.5 In horses, the cervical lobes are small with a large thoracic lobe.5,21 In domestic small animals, the cervical lobes are small (cats) to absent (dogs).5,21 In both cats and dogs, the thoracic lobe is in the ventral cranial mediastinum and extends to the pericardium where in cats it can mold to the pericardial surface.5 The thymus is partially separated into lobules by connective tissue septa that extend from the capsule so that each lobule comprises a central medulla and surrounding cortex.5,21 The cortex is created by epithelial reticular cells and densely packed differentiating precursor T lymphocytes.5,21 Meanwhile, the medulla contains epithelial reticular cells (relatively larger and more prominent when compared to the cortex), Hassall’s corpuscles, interdigitating dendritic cells, and relatively fewer, less densely packed lymphocytes.5,21 The thymus reaches its maximum size at birth and involutes after sexual maturity, although remnants may be retained through adulthood.5,21 Differentiation between normal age-associated involution and disease-associated thymic atrophy requires interpretation of thymic features considering the patient’s age, clinical presentation, and nutritional state.5,21
Thymic disorders include thymic hyperplasia, congenital thymic cysts, inflammatory diseases, hemorrhages and hematomas, neoplasia (i.e., thymic lymphoma and thymic epithelial tumors), and congenital immunodeficiency disorders.5,21 The most common reaction pattern in the thymus is lymphoid atrophy, which can occur secondary to a multitude of underlying diseases processes including physical and physiologic stresses, cachectic states, toxins (lead, mercury, halogenated aromatic hydrocarbons, mycotoxins (i.e., fumonisin B1 & B2 and aflatoxin)), drugs, radiation, neoplasia, and viral infections.5,21 True thymitis is rare but can be seen with certain infectious etiologies (i.e., Neorickettsia helminthoeca [salmon poisoning], Neorickettsia elokominicas [Elokomin fluke fever], epizootic bovine abortion, and porcine circovirus 2).5,21 Thymic hemorrhage and hematomas are more frequently reported in young dogs, for which reported causes include anticoagulant rodenticides, dissecting aneurysm, trauma, and idiopathic/spontaneous.5,21
Tumors primary to the thymus are uncommon and include thymic lymphoma, thymic epithelial tumors, and thymic germ cell tumors.5,7,9,10,20,21 Thymic epithelial tumors (TET) are uncommon cranial mediastinal neoplasms originating from the thymic epithelium and include thymoma, thymic carcinoma, and thymic neuroendocrine tumors.1,5-7,14,15,18,20-26 Thymomas have been reported in numerous species, including dogs, cats, small and large ruminants, horses, pigs, rabbits, ferrets, birds, non-human primates, rodents, a polar bear, and an otter, and are most typically identified in adult to aged animals, without a sex predilection.2,3,5,6,8,14,15,17,18,20-24,26 They are reportedly more common in goats with a 25% incidence reported in a single closed herd of Saanen dairy goats.3,5,6,14,15,17,20,21,24,26 Generally, thymomas are not associated with a specific breed predisposition in domestic small animals but medium to large breed dogs are often affected.15,20 In one retrospective case series, Labrador retrievers and German shepherd dogs were overrepresented.15,20
Thymomas can be incidental but, when present, clinical signs are non-specific and include weight loss, vomiting/regurgitation, respiratory distress, dyspnea, cough, lethargy, exercise intolerance, and edema of the ventral head, neck, and forelimbs.6,15-17,18,20,21,23,24 In cases of cervical thymoma, a visible or palpable subcutaneous mass may be present.6 Associated imaging findings include a soft tissue opacity in the cranial mediastinum, dorsal displacement of the cardiac silhouette and/or trachea, and/or pleural effusion.6,15,26 Advanced imaging (CT or MRI) is the most reliable method to assess tumor invasiveness.6,15,17 Additional clinical signs, gross examination findings, and imaging findings associated with paraneoplastic syndrome(s) may be present.
The most common location of thymoma is the cranial mediastinum, but thymomas have been reported in the neck, thoracic inlet, and rarely in ectopic thymic tissues.6,8,17,20,21,23 Cytologic features indicative of thymoma include lymphocytes (predominantly small T lymphocytes), thymic epithelial cells, and few mast cells, eosinophils, and/or macrophages.15,17,26 As the non-neoplastic lymphoid component exfoliates readily, neoplastic epithelial cells are variably present in cytologic preparations.15,20,24,26 In this case, the predominant cell population on cytology was mildly pleocellular spindle cells mixed with few mast cells, lymphocytes, and hemosiderin-laden macrophages. Initially, these spindle cells were of unclear etiology (neoplastic versus reactive population), but when evaluated along with the histology, they represented neoplastic epithelial cells displaying a spindle morphology.
The histologic appearance of thymomas is diverse due to the range of neoplastic cell morphologies and degree of benign lymphocyte infiltration.1,5,6,14,17,20,23 Additional features inconsistently seen in thymomas include loss of normal thymic architecture, loss of corticomedullary junction distinction, protein-filled cysts, necrosis, and hemorrhages.6,8,20,21,26 Neoplastic thymic epithelial cells can display spindle, oval, and round to polygonal morphologies and exhibit various growth patterns.3,14,17,20,23,26 Typically, neoplastic cells have ill-defined cell margins and single nuclei with vesicular chromatin and up to one prominent nucleolus.3,15,17,20,23,26 An uncommon clear cell variant comprised of large round cells with abundant amounts of clear cytoplasm and prominent cell margins has been described.15,20 In one study, 30.6% of canine cases were inconclusive on cytology and 66.7% of cases had diagnoses with histology alone.24 Thus a small percentage of tumors will require immunohistochemistry for final diagnosis. 24, 26
Thymomas have historically been categorized based on their cellular composition, defined as epithelial-rich, lymphocyte-rich, and mixed/ intermediate types with clear cell, spindle cell, and pigmented variants.8,14,15,17,20,21,23,24 It is rare for thymic epithelial tumors to consist purely of epithelial cells.21 Tumors can be further differentiated as to whether they are encapsulated /non-invasive or invasive.3,15,17,21 Recently, the human WHO classification has been shown to be applicable in canines.21,24 In people the histologic subtype carries prognostic significance, but prognostic significance between subtypes has not been established in veterinary medicine.9,10,20,21,24
The WHO classification is based on epithelium morphology, severity of cellular pleomorphism, and degree of proliferating non-neoplastic lymphocyte; under this scheme, thymomas are classified into A, AB, B1, B2, and B3 type, micronodular, and metaplastic thymomas.2,7,9,10,21,24,25 Type A thymomas contain bland oval to spindle-shaped neoplastic epithelial cells arranged in streaming, storiform, fascicular, pericytomatous, or pseudorosettes with a paucity of T-lymphocytes.7,9,10,21,24,25 Micronodular thymomas likely represent a subvariant of type A tumors where spindle shaped neoplastic epithelial cells are separated by large aggregates of lymphocytes.7,9,10,21 Type A thymomas with atypia exist and represent a separate histologic variant.7,9,10,21 Type AB thymomas comprise similar oval to spindle shaped neoplastic cells but are mixed with a focal to diffuse abundance of non-neoplastic lymphocytes.7,9,10,21,24,25 Polygonal epithelial cells can be present in both type A and AB thymomas.9,10 Type B thymomas contain oval to polygonal neoplastic cells with varying numbers of non-neoplastic lymphocytes, as well as variably present perivascular spaces and Hassall’s corpuscles and are further divided into B1, B2, and B3 types.7,9,10,21,24,25 Type B1 tumors maintain a thymus-like microarchitecture and comprise non-clustered neoplastic cells that lack atypia and are mixed with small numbers of lymphocytes.7,9,10,21,24,25 In Type B2 tumors individualized or clustered oval polygonal neoplastic cells lack atypia and are mixed with large numbers of lymphocytes.7,9,10,21,24,25 Lastly, Type B3 thymomas comprise mild to moderately atypical polygonal neoplastic cells arranged in sheets and contain a paucity of small lymphocytes.7,9,10,21,24,25 Metaplastic thymomas are characterized by islands of polygonal neoplastic cells displaying variable atypia on a background of bland spindle cells.7,9,10,21,24,25 Thymic carcinomas comprise neoplastic thymic epithelial cells that display overt features of malignancy and an invasive growth pattern.7,9,10,20,21,24,25
Molecular profiling in humans shows mirroring of the WHO histological classifications and molecular subgrouping, including A-like and AB-like clusters with frequent GFT2I and HRAS mutations, an intermediate B-like cluster with T-cell signaling profile, and a carcinoma-like cluster with high tumor molecular burden and frequent CDKN2A and TP53 alterations.7 Further, thymic carcinomas and B2 and B3 thymomas showed a higher frequency of large copy number variation involving entire chromosomes.7 The GTF2I gene encodes for the TFII-I protein involved in transcription regulation and, in people, GTF2I mutations appear to play a pivotal role in thymic epithelial tumors and may represent a thymoma-specific oncogene.7,25
Typically, thymomas have a benign biologic behavior and the vast majority are slow growing and heavily encapsulated.3,5,6,14,15,17,18 ,20,21,23,26 However, all thymoma subtypes have the potential to behave aggressively with infrequent local tissue invasion and rare metastasis.24 When metastases occur, they most commonly occur to the lungs and regional lymph nodes.17 With surgical excision, most thymomas have a favorable long-term prognosis.1,6,15,17,21,23,24,26 The results of one case series in goats suggests that anatomic location may be a key factor in influencing survival where the prognosis for cervical thymomas with surgical excision were favorable, while excision of mediastinal thymomas more likely carried a guarded prognosis.6 In small animals, there is limited information regarding radiation as a treatment modality in thymomas with reports suggesting a high overall response rate (50-75%) with radiation therapy alone; however, complete response is reportedly rare.15,17,24 In people, the main prognostic factors described with thymomas are tumor size, tumor spread, resection status, histologic subtype, patient characteristics (i.e., age and comorbidities), and response to treatment.7 Prognostic factors in veterinary medicine vary and can be contradictory between studies, but reported factors associated with increased survival times are surgical excision and absence of megaesophagus; those associated with decreased survival times include presence of a paraneoplastic syndrome, myasthenia gravis (as a single variant), metastases, moderate to marked neoplastic cellular pleomorphism, percentage lymphocyte composition, presence of another concurrent tumor, and higher pathologic stage (based on the Masaoka-Koga Staging Systems).15,17,20,24,26 The Masaoka-Koga Staging System evaluates tumor encapsulation, invasion location, and metastases.3,7,24,26 In humans, the Masaoka-Koga Staging System is significantly associated with survival and decreased survival rates are seen with increasing stage regardless of histologic subtype.7,15,24 In one report in dogs, patients with lower staging (stages I, IIa, and IIb) had significantly longer survival times than those with higher staging (stages III, IVa, and IVb);however, a contradictory study did not find a prognostic significance of between stages in dogs.15,24
Paraneoplastic syndromes are complications of malignancy distant from the primary tumor that in some instances can be more harmful than the direct effects of the neoplasm.4,15,24 These syndromes can aid in tumor identification, as well as function as markers of response to treatment.4 The high rate of paraneoplastic syndromes associated with thymic disorders is related to the crucial function of the thymus in immune tolerance through differentiation, selection, and maturation of T lymphocytes from progenitors.1,5,8,11,20,21,24,26 Naïve, CD34+ and CD7+ T lymphocytes are pluripotent and lack helper, suppressor, and killer functions; these progenitors undergo a sequential pattern of antigen expression as a function of orderly gene rearrangements encoding the T cell receptor (TCR).21 While T lymphocytes traverse from the cortex to the medulla, they undergo differentiation and selection via both positive and negative selection, after which only a small percentage of progenitor T lymphocytes survive.5,21 Positive selection occurs in the cortex, where only lymphocytes that recognize MHC molecules, but not self-antigens, are allowed to mature.5,21 Meanwhile, negative selection occurs at the corticomedullary junction, where lymphocytes that recognize both MHC and self-antigen are removed.5,21 Thymic “nurse cells” produce IL-1 which drives lymphoproliferation.21 Once mature, T lymphocytes leave the thymus, enter the bloodstream, and circulate through secondary lymphoid organs.5,21 Thymomas have been associated with a vast array of paraneoplastic processes with related clinical signs; these include myasthenia gravis, hypercalcemia of malignancy, immune-mediated polymyositis, keratoconjunctivitis sicca (KCS), immune-mediated thrombocytopenia, immune-mediated anemia, granulocytopenia, hypergammaglobulinemia, cardiac myositis with arrhythmias, feline exfoliative dermatitis, erythema multiforme, and T lymphocytosis.2-4,11,13-15,17-21,23,24,26
Disruption of central tolerance has been proposed as one of the mechanisms leading to autoimmunity associated with thymoma.11 Thymic epithelial cells express elevated levels of MHC I and II molecules.20 Aberrant expression of autoantigens and decreased expression of MHC molecules and autoimmune regulator (AIRE) on neoplastic epithelial cells result in defective thymic selection and survival of autoreactive lymphocytes.20 Clinical signs associated with paraneoplastic syndromes may resolve after surgical excision of the mass, but sometimes clinical features of paraneoplastic syndromes can develop after mass removal.2-4,11,13-15,17-21,23,24,26
Myasthenia gravis and hypercalcemia related to parathyroid hormone-related peptide (PTHrP) are the most frequently reported thymoma-associated paraneoplastic syndromes in dogs with reported prevalence of 10-45% (myasthenia gravis) and 30% (hypercalcemia).2,6,14,15,20,26 In this case, myasthenia gravis was suspected based on the clinical presentation but was not confirmed with ancillary testing. The syndrome is classically categorized as either acquired or congenital myasthenia gravis; however, recently alternative terminology has been suggested where myasthenia gravis exclusively refers to acquired forms and the term congenital myasthenia syndromes is proposed for congenital forms.12,18,19,21 Acquired myasthenia gravis can occur with or without thymoma, and is associated with thiourylene medication administration in cats.12
Immune-mediated myasthenia gravis is most commonly due to the production of autoantibodies towards acetylcholine receptors (AChR) leading to destruction of the post-synaptic membrane and reduction of available functional AChR at the neuromuscular junction.2,5,12-14,18-21 Less frequently, autoantibodies targeted against other muscle specific proteins (muscle specific kinase [MUSK], titan, and ryanodine proteins) have been reported. The pathogenesis of autoantibody production with thymoma is not entirely known but thymic myoid cells and dysregulation of T lymphocyte selection is thought to play a role.2,13-15,18-21 Differentiating T lymphocytes recognize autoantigens to AChR or other skeletal muscle specific protein subunits and, due to a defective selection process, they survive and in turn stimulate proliferation and differentiation of B lymphocytes with subsequent autoantibody production.12,18,20,21 At the neuromuscular junction, proposed mechanisms for receptor damage/inactivation include direct damage or formation of cross-linked antibodies leading to receptor internalization.19
The clinical manifestations of myasthenia gravis are divided into focal, generalized, or acute fulminating forms.12,21 Focal manifestations affect an isolated skeletal muscle group outside the appendicular skeletal muscles (i.e., facial, esophageal, pharyngeal, and laryngeal skeletal muscles).12,21 Generalized manifestations are defined as appendicular skeletal muscle weakness with or without facial, esophageal, pharyngeal, or laryngeal skeletal muscle involvement.12 In fulminant forms patients present with an acute, rapidly progressive, and severe form of generalized myasthenia gravis that can progress to respiratory collapse requiring intubation and mechanical ventilation.12
Clinical signs vary between patients and include exercise intolerance, decreased or absent peripheral reflexes, cervical weakness, muscle weakness, flaccid paralysis, episodic collapse, hypersalivation, dysphonia, dysphagia, and regurgitation.2,12,13,18-21,26 Initially enough functional AChRs are present to allow for normal transmission, but with sustained muscle activity the number of available receptors decreases, leading to progressive or episodic weakness and collapse.19 Megaesophagus is a common sequela that predisposes patients to aspiration pneumonia.5,21,26 Diagnosis of myasthenia gravis requires detection of circulating antibodies to AChR, however a subset of dogs with generalized disease will be seronegative.12,13,15,18,20 There is a correlation between fluctuations in autoantibodies and clinical course of disease.18 Clinical responses to administration of an anticholinesterase drug (edrophonium chloride; Tensilon) can be helpful but are not absolutely specific.12,13,15,19,20 Therefore, ancillary testing results should be evaluated in concert with thoracic imaging and clinical signs.12 Complete excision may lead to normalization of neuromuscular junction activity and resolution of clinical signs, but signs of myasthenia gravis can also develop after thymectomy.12,15,18-21
Contributing Institution:
Schwarzman Animal Medical Center
Department of Anatomic Pathology
www.amcny.org
JPC Diagnosis:
Mediastinal mass: Thymoma, type A.
JPC Comment:
The contributor provides an excellent, thorough overview of thymoma, the various applicable classification schemes, and associated paraneoplastic syndromes; it is an enjoyable, informative read and a lily that requires no gilding.
This week’s conference was moderated by Dr. Amy Durham, Professor of Anatomic Pathology at the University of Pennsylvania School of Veterinary Medicine, who opened discussion of this case by noting that the basics of this neoplasm, including tissue identification and cell of origin, are not particularly obvious without the clinical history and diagnostic results provided by the contributor. Conference participants seemed to agree, as, in addition to thymoma, a wide range of differentials were presented, including various sarcomas and vascular entities. The moderator noted that a well-curated immunohistochemistry plan, including vimentin, cytokeratin, and lymphocyte markers, makes the diagnosis. Vimentin is particularly helpful in this case given the spindle-cell morphology of the epithelial cells, which is uncommon in dogs and can easily cause the neoplasm to be misidentified as mesenchymal without immunohistochemistry.
A particularly astute conference participant noted the presence of multifocal pockets of ciliated epithelium within the thymic parenchyma and suggested that these might represente branchial cysts. The moderator noted that branchial cysts, which are persistent remnants of the embryonic pharyngeal pouches, are commonly encountered in the mediastinal tissues and, while interesting, are typically of no moment.
Conference participants discussed the value of the various classification schemes which, as noted by both the contributor and the moderator, currently have no prognostic value. The moderator noted that she typically prefers to diagnose these simply as thymomas, without subclassification, unless the specifics of a particular case warrant emphasizing the tumor’s morphology. Other conference participants shared this approach, but all remained ready to adopt any classification scheme that becomes clinically useful in the future. During discussion of the morphologic diagnosis, however, a resident noted that the WHO classification scheme is exhaustively detailed in the primary veterinary pathology textbooks; therefore, for educational purposes, this delightful and slightly enigmatic neoplasm’s WHO Type A classification was retained in the morphologic diagnosis.
References:
- Agrafiotis AC, Siozopoulou V, Hendricks JNH, et al. Tumor microenvironment in thymic epithelial tumors: A narrative review. Cancers. 2022;14:6082-6093.
- Akiyama T, Morita T, Takimoto Y, et al. Lymphoepithelial thymoma characterized by proliferation of spindle cells in a Samoyed dog. J Vet Med Sci. 2009;71(9): 1265-1267.
- Cavalcanti JV, Maura MP, Montiera FO. Thymoma associated with exfoliative dermatitis in a cat. J Feline Med Surg. 2014;16(12):1020-1023.
- Cullen JM, Breen M. An overview of molecular cancer pathogenesis, prognosis, and diagnosis. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley Blackwell;2017:15-16.
- Durham AC, Boes KM. Bone Marrow, Blood Cells, and the Lymphoid/Lymphatic System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier:2022;856,862-864,878-880, 888.
- Hill JA, Fubini SL, Hackett RP. Clinical features and outcome in goats with thymoma: 13 cases (1990-2014). J Am Vet Med Assoc. 2017;251:829-834.
- Kuhn E, Pescia C, Mendogni P, et al. Thymic epithelial tumors: an evolving field. Life. 2023;13:314-335.
- Li WT, Chang HW, Jeng CR, et al. Concurrent spindle-cell thymoma and thymic cysts in a Barbary sheep (Ammotragus lervia): case report and review of the literature. J Vet Diagn Invest. 2016;28(6):744-749.
- Marx A, Chan JKC, Chalabreysse L, et al. The 2021 WHO classification of tumors of the thymus and mediastinum: what is new in thymic epithelial, germ cell, and mesenchymal tumors? J Thorac Oncol. 2021;17(2):200-213.
- Marx A, Chan JKC, Coindre JM, et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10: 1383-1395.
- Mendoza-Kuznetsova E, Piedra-Mora C, Bizikova P. Comorbidity of ectopic thymoma-associated exfoliative dermatitis and pemphigus foliaceus in a cat. Can Vet J. 2021;62:1067-1070.
- Mignan T, Targett M, Lowrie M. Classification of myasthenia gravis and congenital myasthenia syndromes in dogs and cats. J Vet Intern Med. 2020;34:1707-1717.
- Miller AD, Porter BF. Nervous System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier: 2022;987.
- Moller CA, Bender H. Pathology in Practice. J Am Vet Med Assoc. 2017;250(4): 387-390.
- Robert CS, Casario L, Gaeta CL, et al. Clinical features, treatment options, and outcome in dogs with thymoma: 116 cases (1999-2010). J Am Vet Med Assoc. 2013; 243:1448-1454.
- Rottenberg S, von Tscharner C, Roosja PJ. Thymoma-associated exfoliative dermatitis in cat. Vet Pathol. 2004;41:429-433.
- Serfilippi LM, Quance JL. Pathology in Practice. J Am Vet Med Assoc. 2018;253 (2):173-176.
- Singh A, Boston SE, Poma R. Thymoma-associated exfoliative dermatitis with post-thymectomy myasthenia gravis in a cat. Can Vet J. 2010;51:757-760.
- Valentine BA. Skeletal Muscle. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier; 2022: 1013-1014, 1033.
- Valli VE, Bienzle D, Neuten DJ, Linder KE. Tumors of the Hemolymphatic System. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Wiley Blackwell; 2017:305-308.
- Valli VEO, Kiipel M, Bienzle D. Hematopoietic System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Elsevier Saunders;2016:141-158.
- Welle MM, Linder KE. The Integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier: 2022;1209,1261.
- Wernick MB, Hilbe M, Kaufmann-Bart M, et al. Pathology in Practice. J Am Vet Med Assoc. 2013;243(8):1117-1119.
- Yale AD, Priestnall SL, Pittaway R, et al. Thymic epithelial tumors in 51 dogs: histopathologic and clinicopathologic findings. Vet Comp Oncol. 2022;20:50-58.
- Yang J, Zhang B, Guah W, et al. Molecular genetic characteristics of thymic epithelial tumors with distinct histological subtypes. Cancer Med. 2023;12: 10575-10586.
- Zitz JC, Birchard SJ, Couto GC, et al. Results of excision of thymoma in cats and dogs: 20 cases (1984-2005). J Am Vet Med Assoc. 2008;232(8):1186-1192.