WSC 2022-2023
Conference 21
Case 4
Signalment:
8-month-old male American mink (Neogale vison) (non-Aleutian)
History:
This mink was a non-inoculated experimental control animal. All mink were acclimated to the facility after arrival for a period of 3 weeks prior to initiation of the experiment. No clinical signs were observed in this mink.
Gross Pathology:
Bright red lungs that did not collapse normally.
Laboratory Results:
Other mink from this cohort were PCR positive for Aleutian Mink Disease Virus (AMDV) in lung and spleen samples. Frozen samples from this case were not immediately available for molecular testing at the time of conference submission.
Immunohistochemistry for B cell, T cell, and macrophage markers. Most perivascular mononuclear cells stained with CD3 antibody with immunohistochemistry.
Microscopic Description:
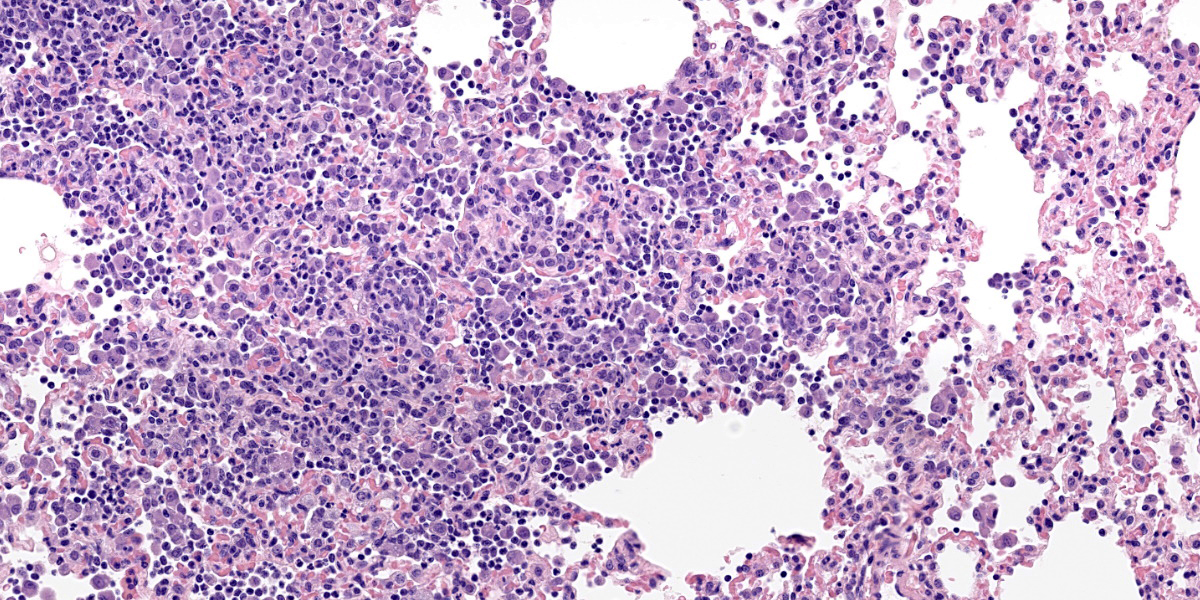
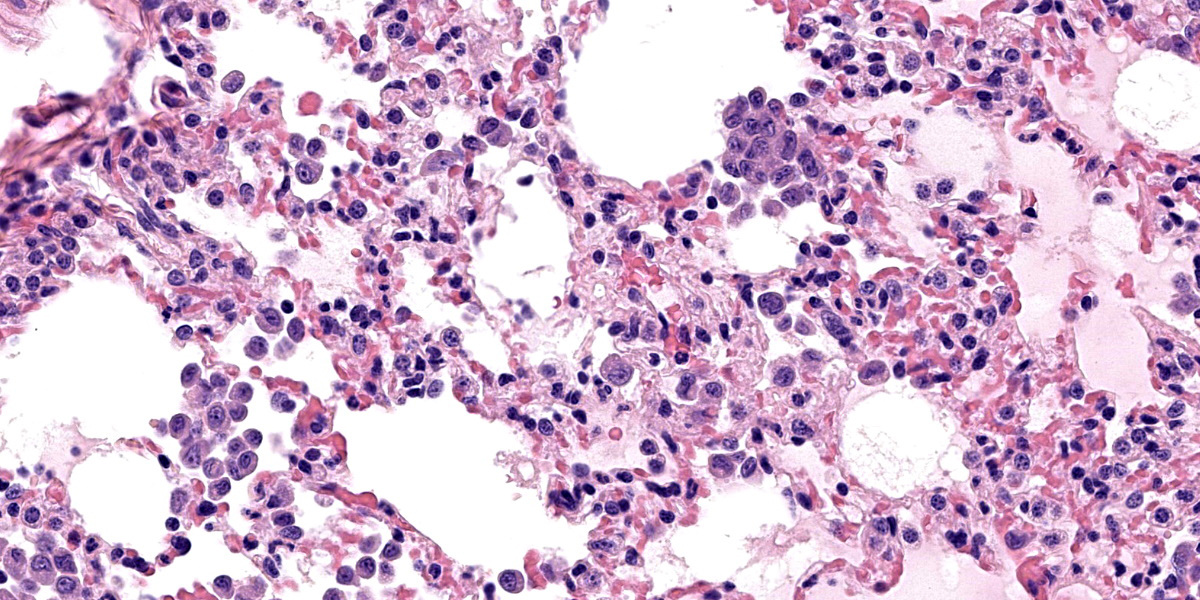
Lung: The alveolar septa are diffusely thickened due to increased numbers of lymphocytes and enlarged round to cuboidal epithelium (type II pneumocyte hyperplasia) lining the alveoli. There are many lymphocytes with fewer plasma cells around pulmonary vasculature. There are multifocal areas of low numbers of neutrophils within alveoli and alveolar septa along with numerous alveolar macrophages. Alveolar macrophages have two distinct morphologies. The first type is 20-35 microns in diameter with a round to reniform 10-20 microns in diameter; the cytoplasm is amphophilic with an eosinophilic perinuclear clear zone. The second population of macrophages have prominent 2–3-micron intracytoplasmic vacuoles. There are occasionally multinucleated macrophages. Alveolar lumens also contain sloughed epithelial cells multifocal areas of eosinophilic proteinaceous fluid. Alveolar septa are lined by a curved cup-shaped eosinophilic material. Capillaries are congested with red blood cells and occasionally filled with dense eosinophilic material that expands the capillary. Near the lung periphery, capillaries are largely devoid of red blood cells.
Pneumocytes occasionally have vacuolated cytoplasm. Rarely within intra-alveolar cells, there are oval to round magenta intranuclear inclusion bodies 3-4 µm in diameter with peripheralized chromatin. There are multifocal areas where alveolar septal walls are absent, and alveoli conjoin (microbullae).
Contributor’s Morphologic Diagnoses:
Interstitial pneumonia, lymphohistiocytic, diffuse, moderate, chronic with perivascular lymphocytes.
Contributor’s Comment:
The microscopic findings are consistent with Aleutian Mink Disease Virus (AMDV). Previous mink from this shipment died acutely upon arrival and were positive for AMDV by PCR in the spleen and lungs and had concomitant severe hepatic lipidosis.
Aleutian mink disease virus (AMDV) is the archetype and prototype member of Amdoparvoviruses that infects domesticated and wild mink. Individual cases of infection with AMDV in other species is reported, but some historic reports did not include evaluation of sequence. The viral family within the carnivores has recently grown, with distinct, host-specific amdoparvoviruses (APVs) discovered in fishers, grey foxes, red foxes, raccoon dogs, red pandas, and striped skunks.8 In AMDV infected mink, the host age and immune status affects the clinical outcome. In mink kits, the disease classically manifests with interstitial pneumonia and viral replication is predominantly within type II pneumocytes.3,7 In adult mink, the virus is found in macrophages in lymphoid tissue. Plasma cells proliferate and infiltrate the liver, spleen, kidneys, and lymph nodes. The disease has also been called mink plasmacytosis because of this. Mink have a hypergammaglobulinemia and develop renal disease characterized by glomerulonephritis and interstitial nephritis. Glomerulonephritis and vasculitis are due to viral-antibody complexes (type III hypersensitivity) in affected mink.3,6
Aleutian mink are named for a hair coat color dilution due to a genetic mutation. The mutation, termed Chédiak-Higashi syndrome, results in defective lysosomes, melanosomes, cytolytic granules, and platelet rich granules.6 Aleutian mink are more susceptible to AMDV and appear to be affected by all isolates or strains; whereas, non-Aleutian mink, may be less susceptible to some virus strains resulting in non-progressive persistent infections.3
Contributing Institution:
National Animal Disease Center
https://www.ars.usda.gov/midwest-area/ames/nadc/
JPC Diagnosis:
Lung: Pneumonia, interstitial, lymphohistiocytic, diffuse, moderate, with type II pneumocyte hyperplasia, peribronchial and perivascular lymphoplasmacytic infiltrates, and intranuclear viral inclusion bodies.
JPC Comment:
As the contributor mentions, a critical component of the pathogenesis of Aleutian mink disease is the exuberant production of antibodies by plasma cells leading to a type III hypersensitivity reaction (immune complex hypersensitivity). These reactions occur when immune complexes (typically containing IgM and IgG) accumulate in tissues, activate complement, and recruit neutrophils, leading to local damage.6 Type III hypersensitivity reactions generally occur when there is slightly more antigen than antibody; if antibody is present in large quantities, the insoluble complex is easily cleared by tissue macrophages and hypersensitivity does not develop.5,6 Complexes may deposit in vessels, joints, glomeruli, or choroid plexus.4,6 Type III hypersensitivity reaction typically occur in diseases that are the result of persistent infection (i.e. Aleutian mink disease), autoimmunity (i.e. systemic lupus erythematosus), or inhalation of environmental antigens (chronic obstructive pulmonary disease).6 The Arthus reaction is a localized, cutaneous version of a type III hypersensitivity reaction that can be induced experimentally via intracutaneous injection of antigen into an animal with a preformed antibody; the resulting reaction produces fibrinoid necrosis in vessel walls and subsequent thrombosis.4
Aleutian mink disease virus is single stranded, negative-sense DNA virus of the genus Amdoparvovirus.2,5 Dr. Pesavento described that diseases associated with other carnivore APVs (Red Panda and Skunk) share many of the features of AMDV in mink, including tissue distribution, but the well described immune complex disease of the infected mink is not present with other APV infections so far.
The amdoparvovirus isolated from endangered red pandas (RPAV) is widespread in captive populations in the United States, with the virus isolated from at least 50% of 104 animals tested.1 Infection appears to be persistent, as well: the virus was consistently isolated from 6 animals serially sampled for up to 6 years.1,2 In the index case, the red panda had virus associated by ISH with significant pyogranulomatous inflammation in multiple sites, including the peritoneum, pancreas, and heart.1 It appears that a subset of pandas is susceptible to viral associated disease, with heavy loads of virus, and lesions consistently in kidneys, but also variably in other tissues (lung, heart, brain, eggs). In pandas positive for the virus, but dead from other causes, virus was identified in normal lymphoid tissues and within normal gastrointestinal mucosa. The intestinal tract, given the consistent shedding and the ISH results, is a likely site of persistence in Red Pandas.
Classically, parvoviruses are thought to infect cells only which are actively replicating (in S-phase, i.e. crypt enterocytes), since the virus requires cellular machinery to replicate, as the contributor mentions. Dr. Pesavento described, however, that human parvovirus B19, the causative agent of fifth disease in humans, induces a DNA damage response (DDR) that facilitates viral DNA replication.
In addition to AMDV, mink are susceptible to another parvovirus, mink enteritis virus, which is closely related to feline panleukopenia virus and an important disease of farmed mink.5,8 Similar to feline panleukopenia, mink enteritis virus targets the enterocytes, causing crypt epithelial necrosis; lymphocytes, causing lymphoid necrosis; and bone marrow progenitor cells, causing leukopenia.8 Curiously, mink enteritis virus is not associated with cerebellar hypoplasia.5
Conference participants discussed euthanasia artifact due to barbiturate salt precipitation as a possible cause for the thickening and eosinophilic debris in the pleura.
References:
- Alex CE, Kubiski SV, Jackson KA, Wack RF, Pesavento PA. Amdoparvovirus infections are prevalent, persistent, and genetically diverse in zoo-housed red pandas (Ailurus fulgens). J Zoo Wildl Medicine. 2022; 53(1): 83-91.
- Alex CE, Kubiski SV, Li L, et al. Amdoparvovirus Infection in Red Pandas (Ailurus fulgens). Vet Pathol. 2018; 55(4): 552-561.
- Bloom ME, Kanno H, Mori S, Wolfinbarger JB. Aleutian mink disease: puzzles and paradigms. Infect Agents Dis. 1994;3: 279-301.
- Kumar V, Abbas AK, Aster JC. Diseases of the Immune System. In: Robbins & Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier. 2021; 210-212.
- MacLachland NJ, Dubovi EJ, eds. Parvoviridae. In: Fenner’s Veterinary Virology. San Diego, CA: Elsevier. 2017; 251-255.
- Snyder PW. Disease of Immunity. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th Philadelphia, PA: Elsevier, Inc. 2022: 320.
- Turner P. Viral Diseases of Mink. The Merck Veterinary Manual 11. Retrieved 6/6/2022, from https://www.merckvetmanual.com/exotic-and-laboratory-animals/mink/viral-diseases-of-mink.
- Williams BH, Burek Huntington KA, Miller M. Mustelids. In: Terio KA, McAloose D, St. Lger J, eds. Pathology of Wildlife and Zoo Animals. San Diego, CA: Elsevier. 2018; 296.