Signalment:
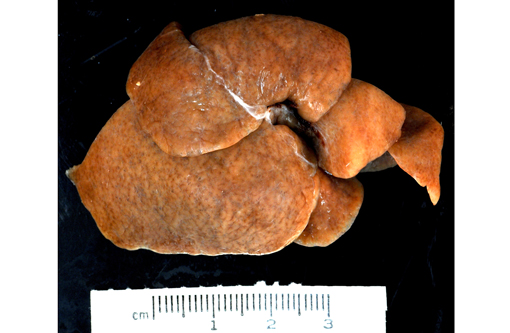
Gross Description:
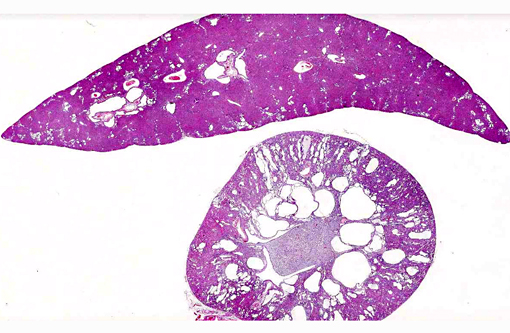
Histopathologic Description:
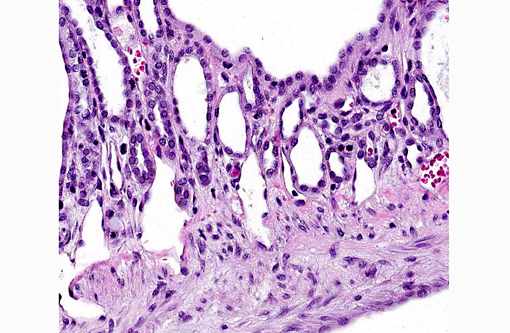
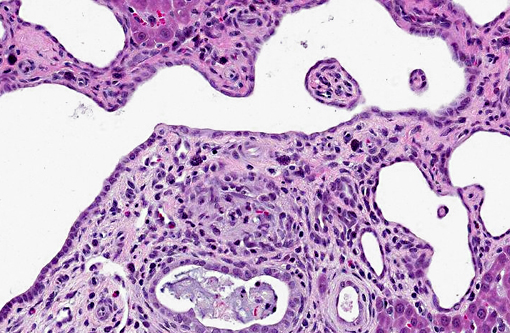
Liver: There are multiple individual to coalescing spaces that were lined by variably attenuated cuboidal epithelial cells (cysts) and surrounded by a band of collagenous tissue. In the adjacent hepatic parenchyma, there was slight atrophy of hepatic cords, proliferation of bile ducts, and minimal mixed inflammatory cell infiltrate in periportal regions.
Morphologic Diagnosis:
1. Kidney: Severe multifocal renal tubular dilatation and ectasia with mild interstitial fibrosis.
2. Liver: Marked multifocal biliary duct hyperplasia and ectasia with mild portal fibrosis.
Lab Results:
Condition:
Contributor Comment:
In humans, there are two types of PKD: autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD). In the more common form, ADPKD, the parenchyma is extensively replaced by cysts that originate from all segments of the nephron, collecting tubules, and ducts. In humans with ADPKD, there is an association with cysts in other organs, most often the liver. Other abnormalities that can be coupled with ADPKD include: cardiac valvular anomalies, intracranial aneurysms, and colonic diverticula. In ARPKD, cysts arise from only dilated collecting tubules and ducts and in most cases there is also biliary dysgenesis and hepatic fibrosis.(1,3)
In veterinary medicine, PKD has been recognized in many species including horses, pigs, lambs, calves, dogs, cats, and rodents. In domestic animals, PKD is most often consistent with ARPKD in that the disease manifests as stillbirths or death within the first few weeks of life due to renal failure. Syndromes resembling both recessive and dominant PKD have been described in domestic dogs and cats.(4,7)
Carolis disease is a rare inherited disorder characterized by dilation of the intrahepatic bile ducts which is associated with liver failure and polycystic kidney disease. In the simple form, only bile ducts are affected. The more complex form, also known as Caroli Syndrome, is also linked with portal hypertension and congenital hepatic fibrosis. The differences between the causes of the two forms have not yet been discovered.(2,9)
Recently, a PKD rat, which is a spontaneous mutant derived from a colony of crj:CD rats, was found to also have polycystic lesions in the liver. Because this may represent an animal model of Carolis disease, it has been well characterized and studied as a model of ADPKD. The mutation arose spontaneously, and initial analysis indicated inheritance as an autosomal recessive trait.(2,8)
The findings presented in this write up describe a case of polycystic kidney disease in a Sprague Dawley rat with involvement of the liver. The involvement of multiple organs suggests that the lesions resulted from a genetic or developmental process rather than an acquired process. Furthermore, the subclinical nature of the disorder and the large size of the kidneys at the time of necropsy suggest that the renal disease was progressive in nature.
JPC Diagnosis:
1. Kidney, tubules: Ectasia, multifocal, marked, with tubular degeneration, loss and mild lymphoplasmacytic interstitial nephritis.
2. Liver, bile ducts and ductules: Ectasia, diffuse, moderate to severe, with biliary ductular reaction.
Conference Comment:
In contrast, polycystic kidneys contain numerous cysts involving multiple nephrons.(7) In veterinary species, inherited PKD can be autosomal dominant or autosomal recessive; lesions are generally bilateral and cysts may affect any part of the nephron. ADPKD is described in bull terrier dogs and adult Persian cats. It can affect both proximal and distal convoluted tubules and typically results in chronic nephritis and renal failure.(4) ADPKD may be associated with mutations of PKD1 and/or PKD2 genes, which result in defective polycystin 1 and/or polycystin 2, respectively.(4) Polycystin 1 is a component of desmosomes that is important in cell adhesion and signaling; its loss may ultimately result in impairment of normal tubulogenesis.(7) Polycystin 2, on the other hand, functions as a plasma membrane calcium channel.(7) ARPKD, reported in Persian kittens, sheep, lambs, and West Highland white and cairn terrier puppies, is caused by a mutation on the PKHD1 gene encoding fibrocystin, which is a receptor protein.(5) In adult Persian cats and West Highland white terrier puppies with PKD, there are often concurrent hepatic cysts.(4) Congenital PKD, with inheritance that is not fully characterized, occurs in pigs, lambs, calves, kids, puppies, kittens and foals. The most common manifestation of this condition is stillbirth or neonatal/infant death.(5)
PKD has also been reported in Brazilian agoutis (Dasyprocta leporina), springboks (Antidorcas marsupialis), an adult raccoon (Procyon lotor), rhesus macaques (Macaca mulatta), slender lorises (Loris lydekkerianus), and a stillborn white-tailed deer (Odocoileus viriginianus).(5) Two separate conditions resulting in polycystic kidneys are reported in goldfish. Infection with the myxosporidian protozoan Mitraspora cyprini causes a condition known as kidney bloater disease, characterized by marked renal tubular hyperplasia and ectasia, with sparing of the glomeruli. Conversely, goldfish polycystic kidney disease primarily involves glomeruli. It is distinguished by severe dilation of the subcapsular space, with the thin connective tissue wall of Bowmans capsule overlying a layer of squamous parietal epithelium. The etiology of this lesion is unknown.(6)
References:
2. Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim. 2000;49(1):51-55.
3. Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon. Review.1995;41(11):693-765.
4. Maxie MG, Newman SJ. Urinary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. St. Louis, MO: Elsevier Limited; 2007:439-444.
5. Muller DWH, Szentiks CA, Wibbelt G. Polycystic kidney disease in adult Brazilian agoutis (Dasyprocta leporina). Vet Pathol. 2009;46(4):656-661.
6. Munkittrick KR, MOccia RD, Leatherland JF. Polycystic kidney disease in goldfish (Carassius auratus) from Hamilton Harbour, Lake Ontario, Canada. Vet Pathol. 1985;22(3):232-237.
7. Newman SJ. Urinary system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2007:618-620.
8. Sanzen T, Harada K, Yasoshima M, Kawamura Y, Ishibashi M, Nakanuma Y. Polycystic kidney rat is a novel animal model of Caroli's disease associated with congenital hepatic fibrosis. Am J Pathol. 2001;158(5):1605-1612.
9. Taylor AC, Palmer KR. Caroli's disease. Eur J Gastroenterol Hepatol. 1998;10(2):105-108.