Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 24
29 April, 2020
CASE II: Case 2 (JPC 4048931). Tissue from a horse (Equus caballus).
Signalment: 7 month-old, male, thoroughbred, horse (Equus caballus)
History: Foal tissue sample were submitted from a farm with a herd of about 300 horses of different ages and sexes. Foals were weaned between four to seven months of age. After weaning, all foals were grouped in batches of about 35 animals based on age. The facilities where the foals were daily handled were the same used for the management of other animals of different ages.
Every year cases of diarrhea were reported with variable severity and
characteristics in foals of different ages. Thirty nine foals, nine from
generation 2011 and 30 from generation 2012, showed clinical signs of pasty
diarrhea evolving to liquid diarrhea. The body temperature varied between 39.5
to 41.0°C within 48 hours, in addition to lack of appetite and dehydration. In
foals with three to six months of age, hypoproteinemia associated with
submandibular edema were frequently observed. The duration of the clinical
signs was from a few days to a few weeks.
Seven of 39 foals had clinical signs of diarrhea and three of these animals
died. A seven month-old foal died four days after the onset of clinical signs
and intestinal samples were submitted to the Laboratory.
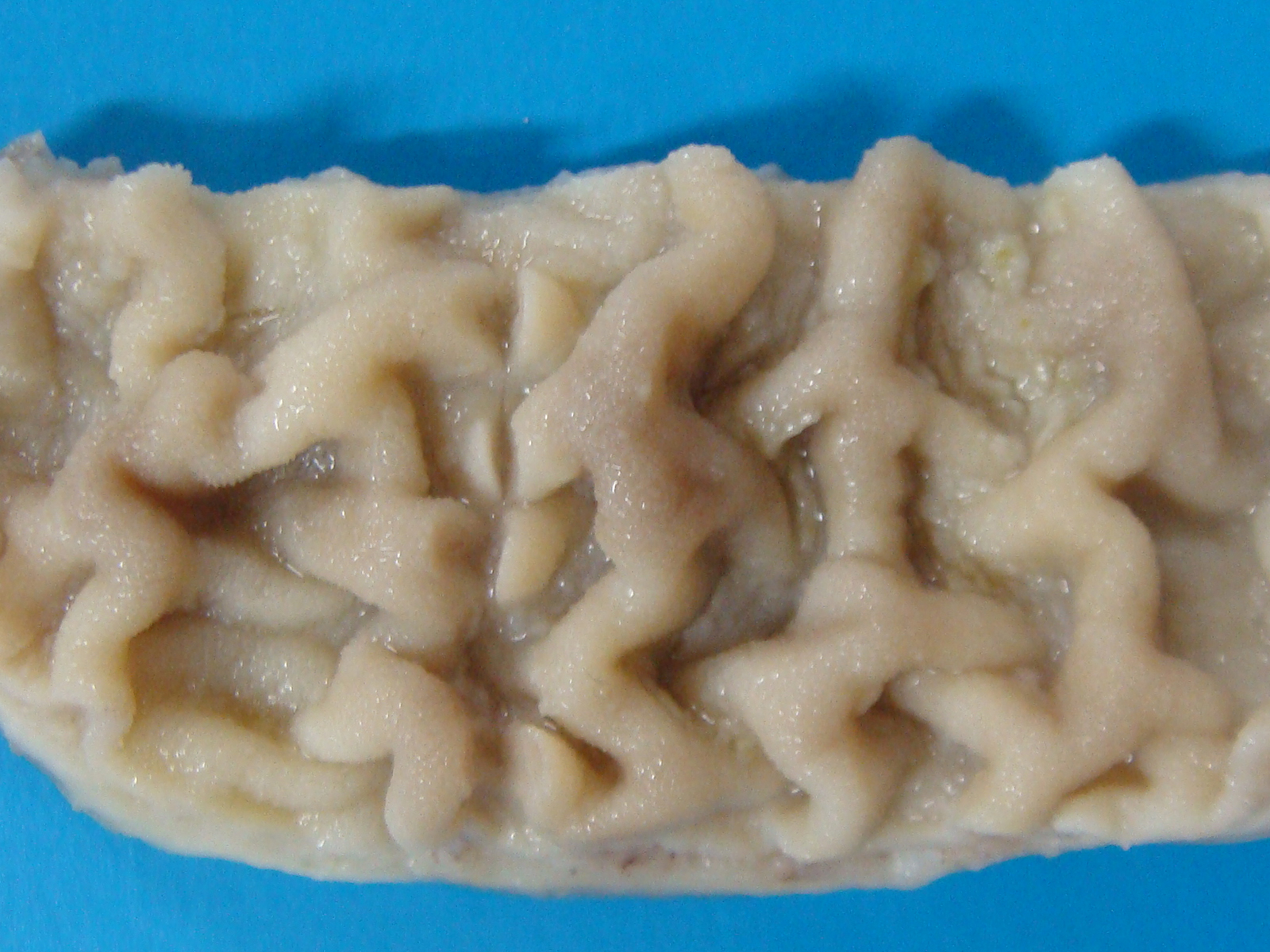
Gross Pathology: Grossly, large amount of blood-tinged fluid was observed in the peritoneal cavity. The serosa of the small intestine was hyperemic. Thickening of the intestinal wall was associated with a clear corrugation and thickening of the mucosa folds (Figure 1) with consequent reduction of intestinal lumen.
Laboratory results: Eleven fecal and serum samples from this group of foals were also submitted to the laboratory. All samples were negative for the detection of Cryptosporidium, Salmonella spp. and, Clostridium perfringens. Three foals were seropositive for Lawsonia intracellularis (1:60 ? Immunoperoxidase monolayer assay). All foals were negative for L. intracellularis by PCR in fecal samples.
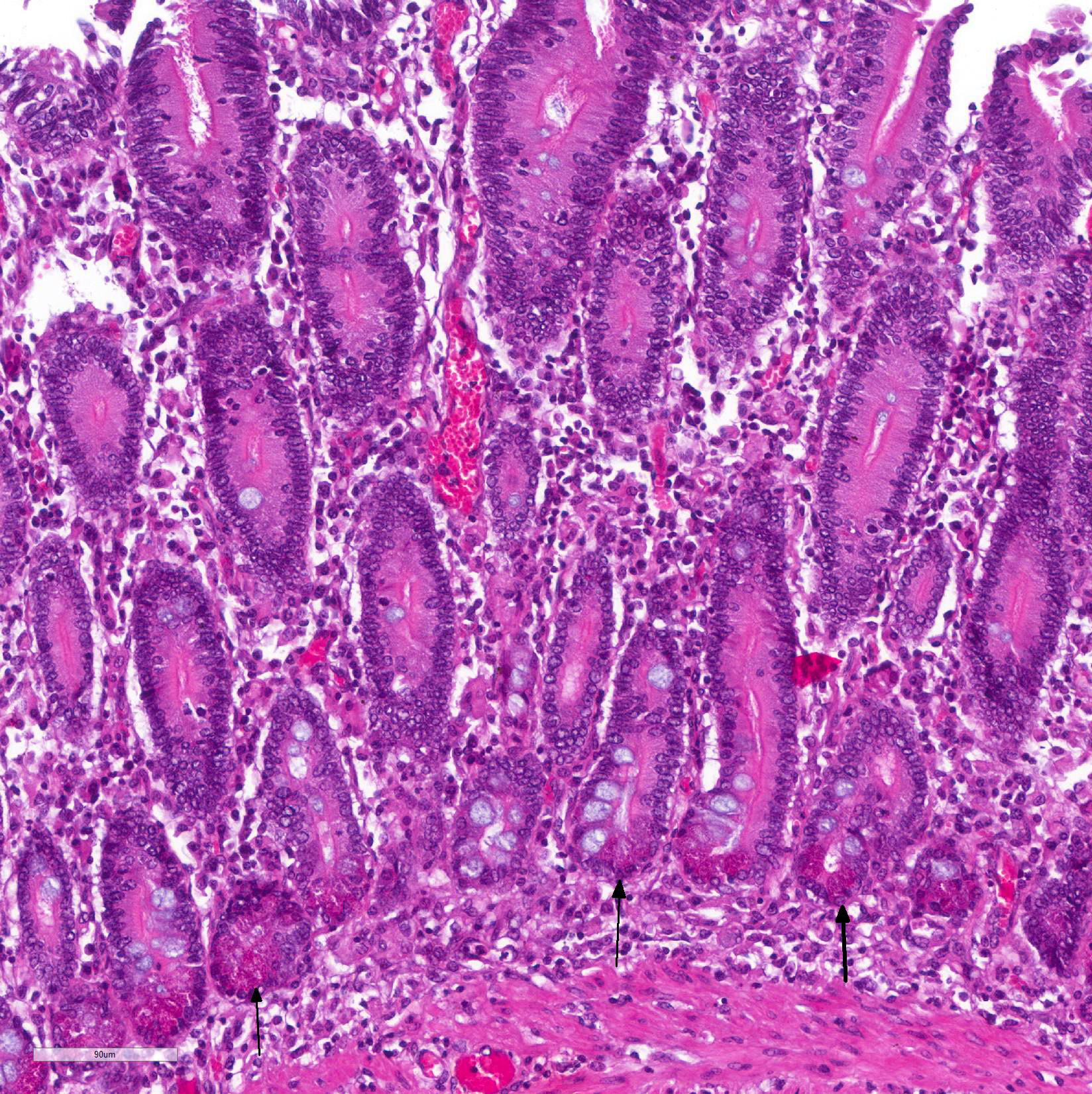
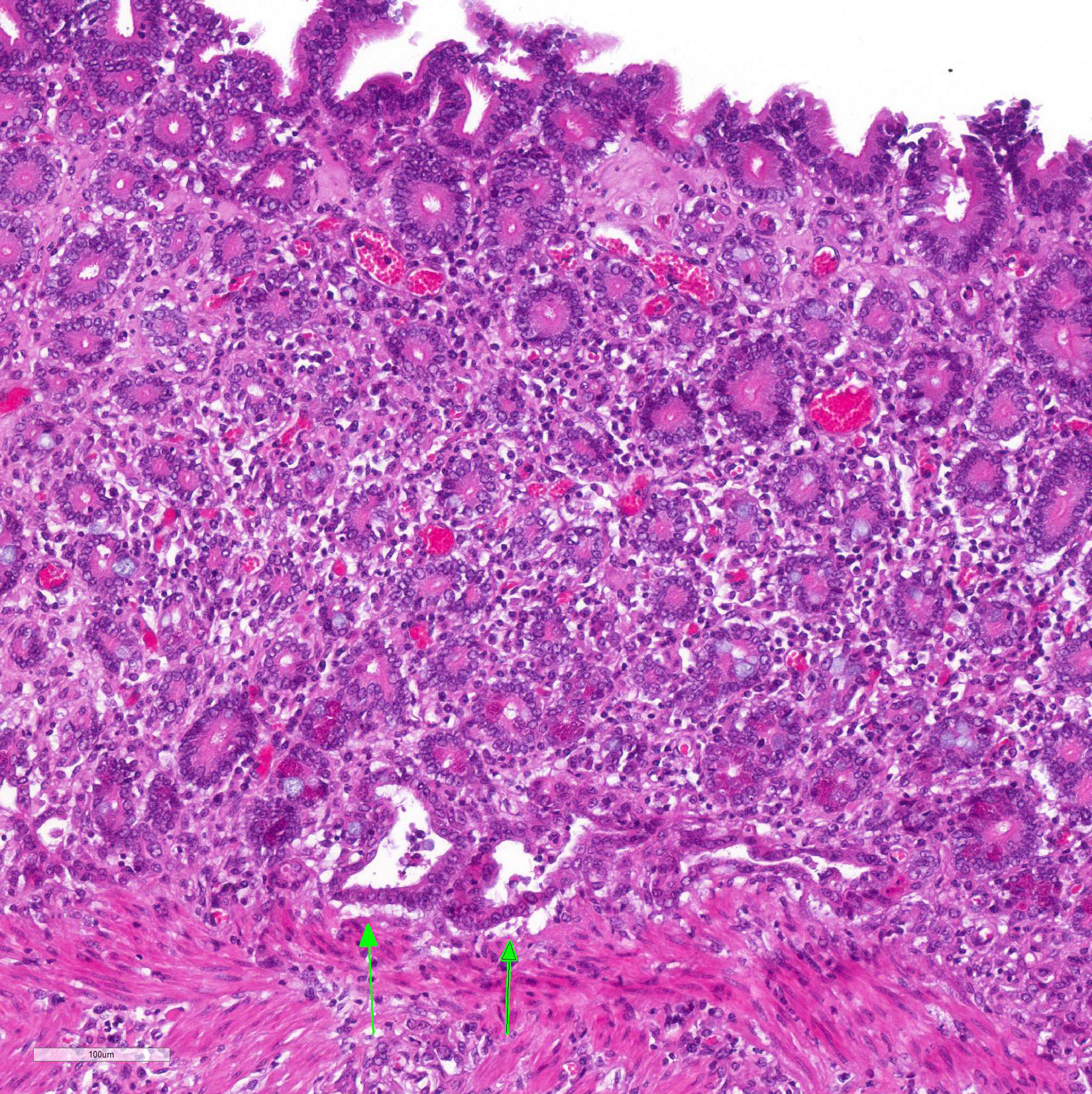
Immunohistochemistry staining using L. intracellularis specific antibodies demonstrated antigen labeling at the cytoplasmic apex of enterocytes and inside macrophages (Figure 3) in the lamina propria of the duodenum, ileum and large intestine.
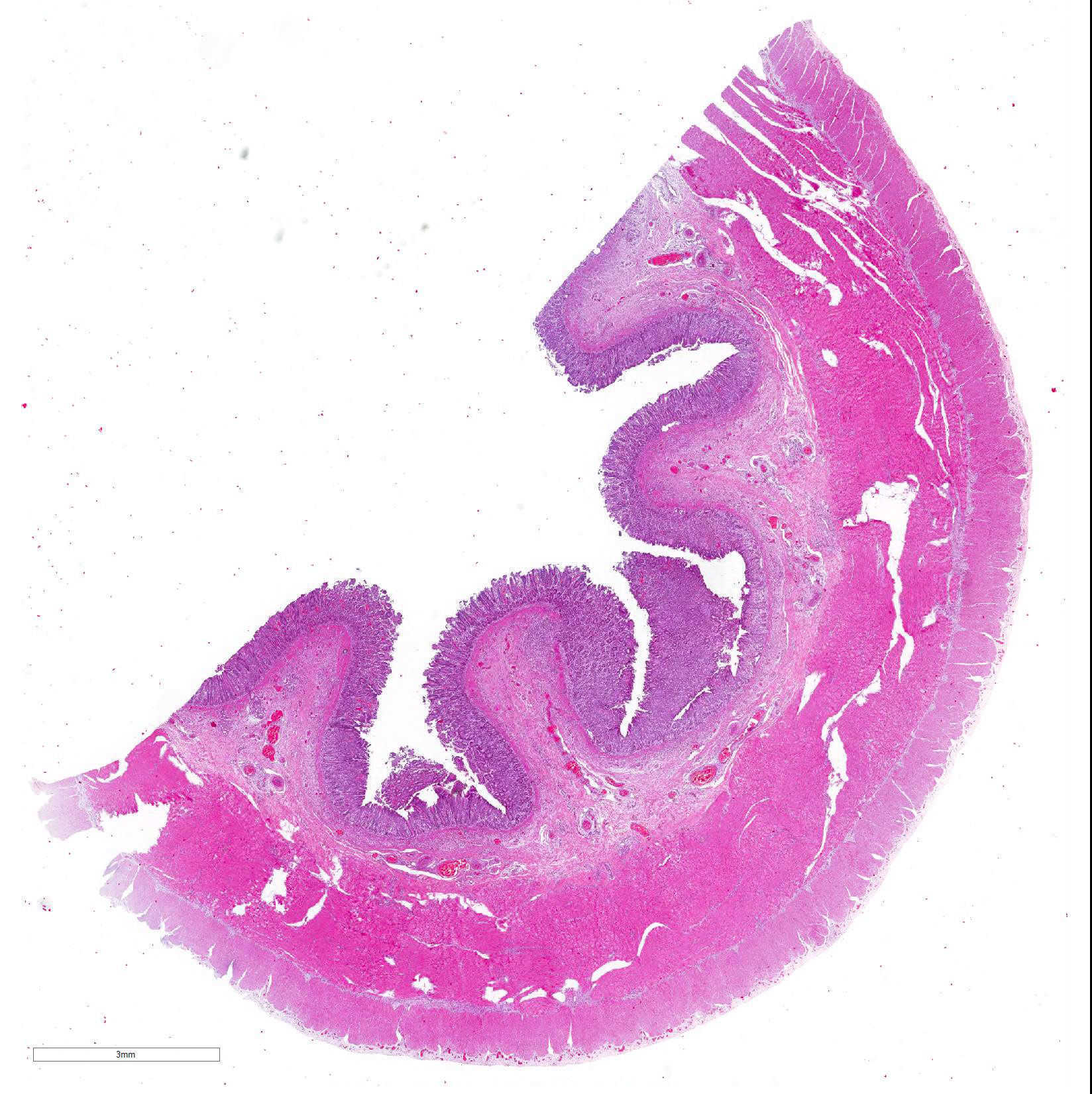
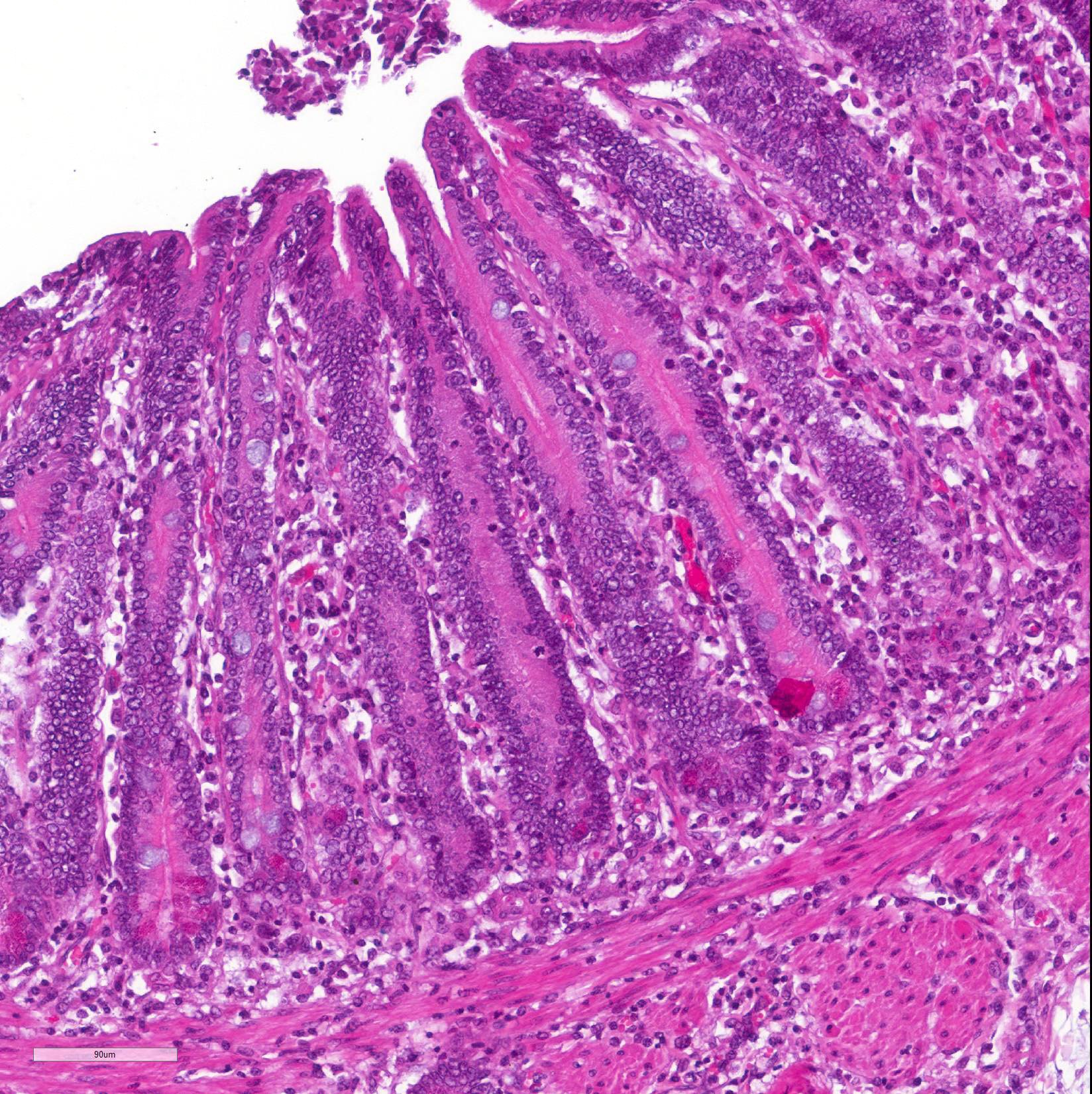
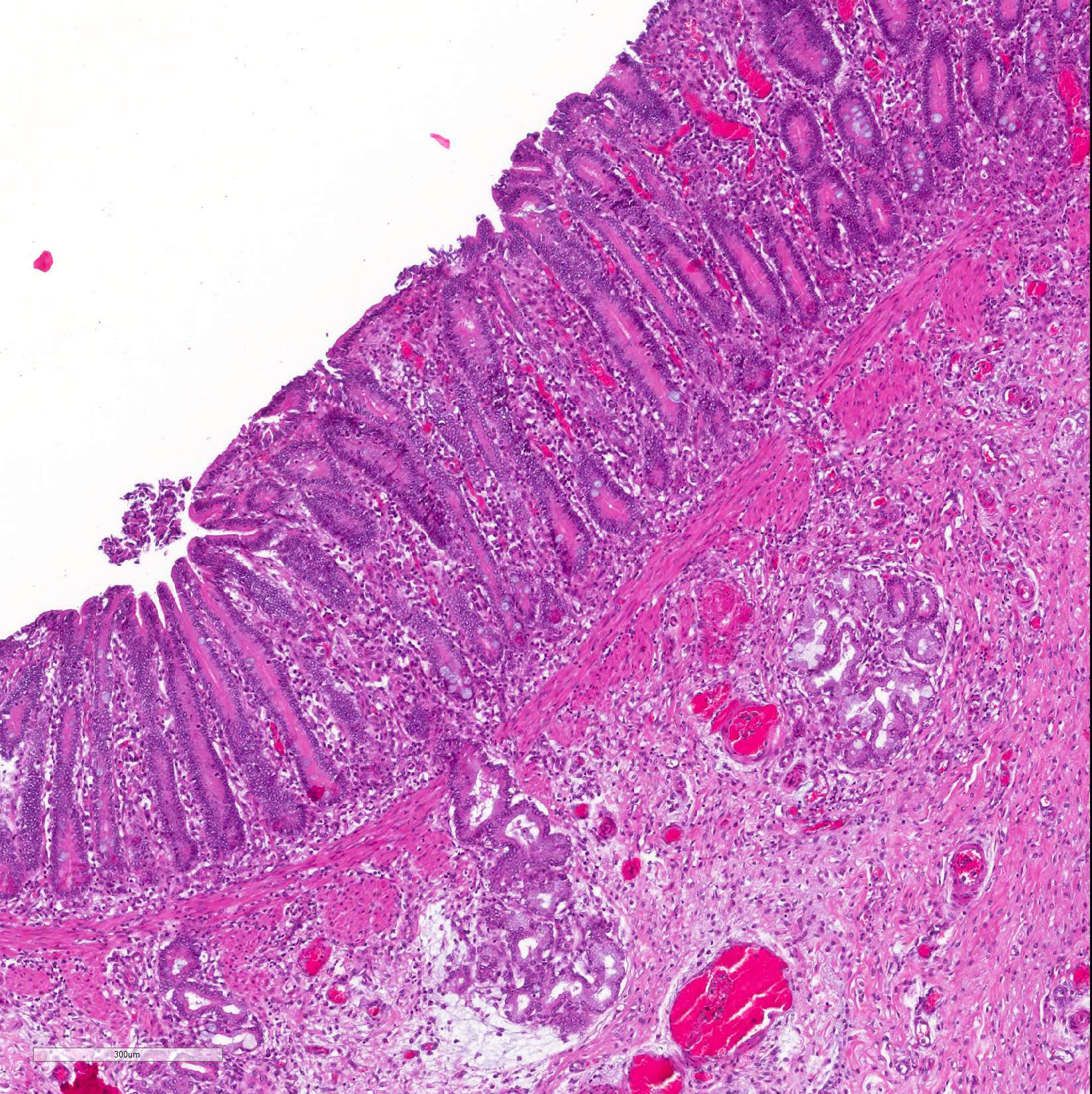
Microscopic Description: Histological analysis of the duodenum, jejunum, ileum and large intestine demonstrated enterocyte hyperplasia of the crypts associated with intense diffuse marked decrease in the number of goblet cells (Figure 2). Rare crypts were dilated and the lumen filled with cell debris and neutrophils (crypt abscess). In addition, crypts were present in some areas of the submucosa.
Contributor Morphologic Diagnosis:
Small intestine, horse: Moderate diffuse proliferative enteropathy with mild multifocal adenomatosis.
Contributor Comment: Reported clinical signs, gross and microscopic findings, all consistent with the literature,11,13 associated with IHC positive signal for L. intracellularis allowed us to reach the diagnosis of equine proliferative enteropathy (EPE). Unlike pigs, in which lesions and immunostaining are more concentrated in the final third of the small intestine, in horses they can also be found in the duodenum,9 as reported in this case. The lesions of the large intestine are less frequent, but in our case, histologic lesions compatible with EPE were also found in colon.
Affected animals had ages ranging from a few days to 21 months of age, however,
diarrhea associated with submandibular edema (consequent of the
hypoproteinemia) was observed more frequently in foals from three to six months
old, the age group in which these animals are more susceptible and affected by L.
intracellularis.8,9 This predisposition is probably associated
with the decline of maternal antibodies,7 as well as stressors such
as weaning, the move to new paddocks and barns, worming and/or vaccination
programs and/or early conditioning programs,4 which were reported in
this farm.
Animals with hypoproteinemia were treated with erythromycin, with clinical improvement in some of these animals. Treatment with erythromycin was effective for EPE in other reports2,8 and is considered one of the treatments of choice for suspected cases of the disease. Disease progression varies from days to weeks, and the prognosis is good, since timely diagnosis and appropriate treatment are performed. Late diagnosed or untreated cases lead to death.10
Seropositivity for L. intracellularis in animals negative by PCR in fecal samples can be explained by the sensitivity of the last technique and/or by the course of the infection. There are many PCR inhibitors in fecal material that reduces the sensitivity of the technique, as a result, the amount of shed bacteria in the feces could be below the detection threshold. In addition, serum antibodies against L. intracellularis last much longer then the bacteria fecal shedding.10
Contamination colts of this report may have occurred through the feces of other positive horses due to daily use of the same facilities for all ages. L. intracellularis subclinical infection seems to be common in horses and fecal shedding of viable bacteria can a source of infection for susceptible foals.5,11 Wild and domestic animals like dogs, cats, opossums (Didelphis spp.), bush dog (Canis thous) sighted on the property can also be sources of contamination. Bacterial DNA has been detected in the feces of domestic and wild animals trapped in farms with the occurrence of EPE.11
The EPE is present in horse farms in Brazil and despite the report of a clinical case of the disease6 and the detection of L. intracellularis in feces and serology in foals in other Brazilian states,5 EPE remains neglected in the differential diagnosis with other enteric disease in foals.
Contributing Institution:
Veterinary School. Universidade Federal de Minas Gerais. (www.vet.ufmg.br)
JPC Diagnosis: 1. Small intestine: Enteritis, proliferative and histiocytic, diffuse, marked with crypt abscesses and submucosal edema.
2. Mesentery: Fat atrophy, diffuse, moderate with lymphohistiocytic steatitis.
JPC Comment: Lawsonia intracellularis is a
gram-negative, non-sporeforming, obligate intracellular bacterium named after
Dr. Gordon Lawson, a long-term researcher at the University of Edinburgh on
porcine proliferative enteropathy and the first to grow the bacterium in pure
culture in 1993. This curve-shaped bacterium lives within the apical cytoplasm
of a number of mammalian species, as well as chickens and ostriches, but has
not been identified as an infectious agent in humans. A list of species which
it has been identified in includes ferrets, swine, horses, dogs, rats, sheep,
deer, ratites, nonhuman primates, and guinea pigs.14 There are a
number of subtleties of infection between species, with ferrets affected in the
colon rather than small intestine, and with species like the rabbit and horse
having a more profound inflammatory component. Marked glandular proliferation
of epithelium (historically referred to as ?adenomatosis?) characterizes the
infection in hamsters and some ages of swine. Cross-species infections are
often unpredictable ? for example, hamsters may be infected with swine isolates
(but not horse isolates) while rabbits may be infected with horse-derived
strains, but not those of swine).12,14
The bacterium possess a single flagellum which provides an important boost to
pathogenicity by allowing it to penetrate the mucus layer in the early stages
of infection. Internalization of the bacilli within apical enterocytes occurs
within 3 hours, but as of yet, the events associated with this important step
have not been characterized.14 The bacilli induces cellular
proliferation of crypt epithelium, and lives free within the cytoplasm, undergoing
binary fission and replication itself within 2-6 days. Infected cells may
release bacilli into the crypt lumen via balloon-like cytoplasmic protrusions,
and these bacilli can then infect adjacent enterocytes.14
In horses, the disease is referred to as equine proliferative enteropathy
(EPE). Primarily affecting foals around weaning (2-8 months), it may also be
seen in adult animals. Weight loss, hypoalbuminemia, peripheral edema, colic,
diarrhea and leukocytosis are seen (but may also be seen in other
gastrointestinal diseases of young horses such as infection with Parascaris
equorum and cyathostomiasis), and ultrasound reveals prominently thickened
loops of small intestine (resulting from the mucosal thickening and submucosal
edema3 evident on this slide). Real time PCR is used to identity the
bacterium in feces and is a very useful antemortem diagnostic test. Affected
horses shed bacteria for 14-21 following active infection, as compared to pigs,
which shed bacilli up to 12 weeks. (This is why subclinical infections in the
pig are much more readily identified than in horses).14Identification
of bacilli within crypt enterocytes may be done at autopsy, but some cases may
yield negative results in PCR-positive animals.3
While PPE is considered a proliferative and inflammatory intestinal disease resulting I ill thrift and hypoproteinemia, a 2013 report by Arroyo et al.1 described an outbreak of ulcerative and necrohemorrhagic disease in weanling foals more reminiscent of the hemorrhagic enteropathy seen as a variant of L. intracellularis infection seen in swine (known as porcine hemorrhagic enteropathy.) These foals, in addition to ulceration, hemorrhage, and the presence of the organism in 3 of 5 cases) failed to demonstrate the marked crypt hyperplasia which is common in horses and most other species. The two other foals in this group were negative for organisms on WS and immunohistochemistry, but were PCR-positive on antemortem fecal samples.1
Another mystery is how foals are infected. Exposure to swine is uncommon
in horse farms today. One recent study examined rodents and stray cats in the
vicinity of horse farms and found a 28% per cent detection rate for cats (via
serology and PCR) and variable but lesser rates for a range of rodents. The
range of infected animals varied greatly between farms, but suggests wild
rodents or other mammals as potential sources on infection.7
References:
1. Arroyo LG, ter Woort F, Baird JD, Tatiersky L, DeLay J, van Drewuel T. Lawsonia intracellularis-associated ulcerative and necrohemorrhagic enteritis in 5 weanline foals. Can Vet J 2013; 54:853-858.
2. Bihr TP. Protein-losing enteropathy caused by Lawsonia intracellularis in a weanling foal. Can Vet J. 2003;44:65-66.
3. Bohin AM, Olsen SN, Laursen SH, OHman A, van Galen G. Lawsonia intracellularis-associated equine proliferative enteropathy in Danish weanlig foals. Acta Vet Scand 2019; 61:12 doi 10.1186/s13028-019-0447-3.
4. Frazer ML. Lawsonia intracellularis infection in horses: 2005?2007. J Vet Intern Med. 2008;22:1243?1248.
5. Guimarães-Ladeira CV, Palhares MS, Oliveira JS, et al. Shedding and serological cross-sectional study of Lawsonia intracellularis in horses in the state of Minas Gerais, Brazil. Equine Vet. J. 2009;41(6):593-596.
6. Guttmann PM, Viscardi V, Lessa DAB, et al. Equine Proliferative Enteropathy Caused by Lawsonia intracellularis in a Foal in Brazil. J. Equine Vet. Science. 2014;34:701-703
7. Hwang JM, Seo MJ, Yeh JY. Lawsonia intracellularis in the feces of wild rodents and stray cats captured around equine farms. BMC Vet Res, 2017; 13:233 doi 10.1186/s/s12917-017-1155-8
8. Lavoie JP, Drolet R, Parsons D, et al. Equine proliferative enteropathy: a cause of weight loss, colic, diarrhea and hypoproteinaemia in foals on the breeding farms in Canada. Equine Vet J. 2000;32(5):418-25.
9. Pusterla N, Gebhart C. Lawsonia intracellularis infection and proliferative enteropathy in foals. Vet Microbiol. 2013;167(1-2):34-41.
10. Pusterla N, Higgins JC, Smith P, et al. Epidemiological survey on farms with documented occurrence of equine proliferative enteropathy due to Lawsonia intracellularis. Vet Rec. 2008;163(5):156-158.
11. Pusterla N, Wattanaphansak S, Mapes S, et al. Oral Infection of Weanling Foals with an Equine Isolate of Lawsonia intracellularis, Agent of Equine Proliferative Enteropathy. J Vet Intern Med 2010;24:622?627.
12. Sampieri F, Vannucci FA, Allen AL, Pusterla N, Antonopoulos AJ, Ball KR, Thompson J, Dowling PM, Hamilton DL, Gebhart CJ. Species specificity of equine and porcine Lawsonia intracellularis isolates in laboratory animals. Can J Vet Res 2013: 77:261-272.
13. Vannucci FA, Pusterla N, Mapes SM, et al. Evidence of host adaptation in Lawsonia intracellularis infections. Vet Res. 2012;20:43-53.
14. Vannucci FA and Gebhart CJ. Recent advances in understanding the pathogenesis of Liawonia intracelullaris infections. Vet Pathol 2014; 51(2) 465-477.