Signalment:
4.5-year-old spayed female domestic ferret,
Mustela putorius euro.The ferret was presented as an emergency to a local veterinary clinic for dyspnea. The ferret was treated with intravenous fluids, Baytril, Cefazolin,
Amoxicillin and oxygen
Gross Description:
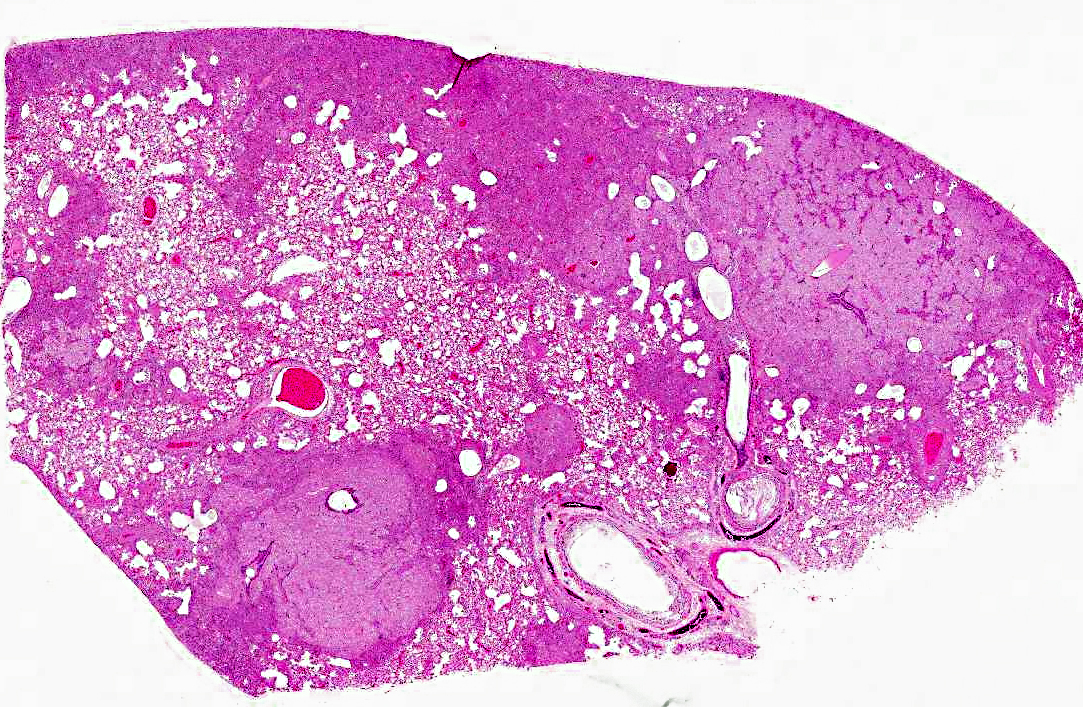
All lung lobes were similarly affected: pale, mottled, with a firm texture, and patchy red areas on cut section. Mediastinal lymph nodes were enlarged.
Histopathologic Description:
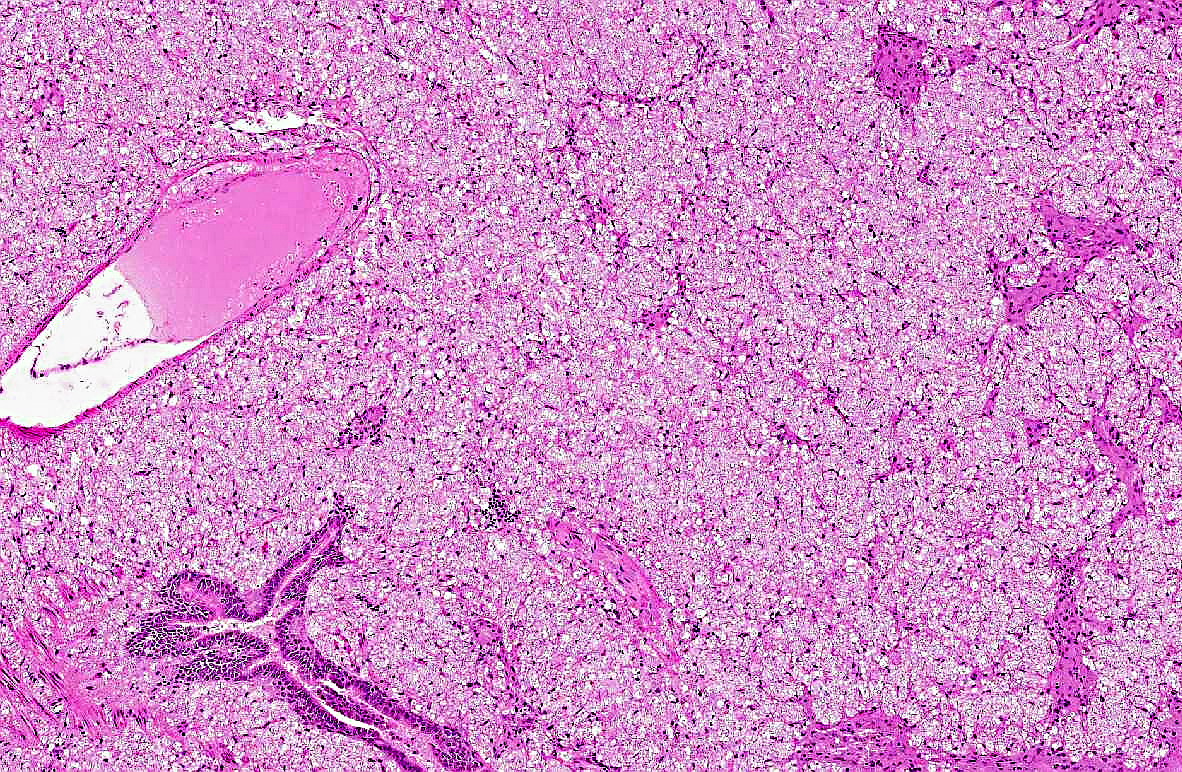
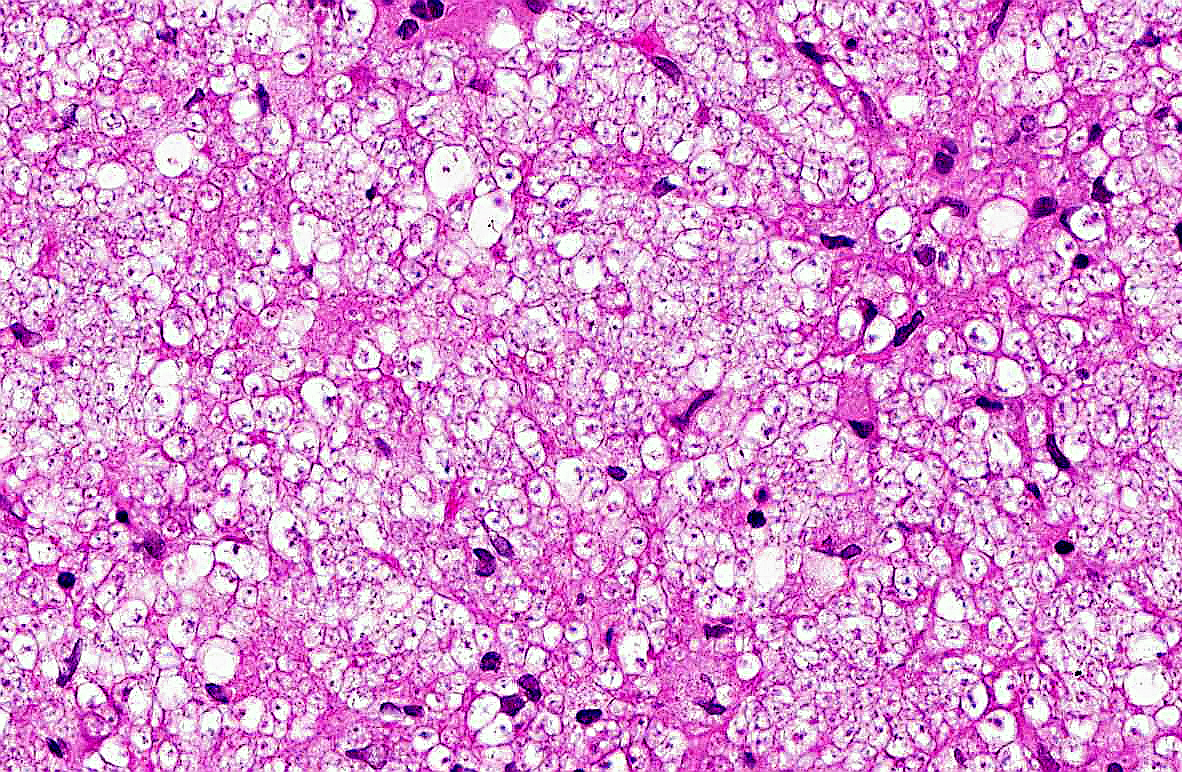
Pulmonary architecture is distorted by locally extensive, alveolar infiltrates of eosinophilic foamy material, which on higher power
examination is composed of myriad approximately 4-10 micron diameter discrete round or oval structures with thin eosinophilic outlines (which stain with
Gomoris methenamine silver) and unstained content except for one or more tiny 0.5 micron central basophilic bodies. These infiltrates are located
predominantly in subpleural, peribronchiolar, perivascular and intramural vascular locations. Surrounding these areas is a more generalized alveolar
infiltrate of large foamy macrophages, many of which contain similar organisms, admixed with moderate numbers of multinucleated giant cells, and lesser
numbers of lymphocytes, plasma cells, neutrophils, with alveolar edema and focal fibrin exudation.
Similar infiltrates were present in the enlarged mediastinal lymph nodes and spleen (sections not provided).
Morphologic Diagnosis:
Severe, subacute granulomatous interstitial pneumonia with infiltrates of organisms morphologically consistent
with
Pneumocystis.
Lab Results:
PCR for influenza virus was negative.
Condition:
Pneumocystis spp.
Contributor Comment:
Pneumocystis spp. are inhabitants of the lung of various domestic and laboratory animal species including ferrets, pigs, foals, dogs, rats, mice, non-human primates as well as humans.(1,2) Originally thought to be a protozoan, Pneumocystis is now classified as a fungus, likely an early divergent lineage of the genus
Ascomycetes.(1)
The organism is considered an opportunistic pathogen, and development of clinical disease usually requires immunosuppression, either by concurrent disease or
the use of immunosuppressive drug therapy.
There are significant genomic, karyotypic, isoenzymatic and antigenic differences among the
Pneumocystis species isolated from different animal
hosts, and there appears to be close host specificity.(1) The taxonomy of these organisms has undergone significant changes in the last decade, and the
organisms originally classified as
Pneumocystis carinii include various divergent strains now recognized as unique species, including
Pneumocystis jirovecii which
colonizes humans,
P. wakefieldiae which together with the original
P. carinii has been found in the Norway rat,
P. murina in laboratory mice and
P. oryctolagi in Old World rabbits. Genetically distinct populations of
Pneumocystis have been also been recognized in the ferret, and may represent multiple separate species, although these have not yet been completely characterized.(6)
Pneumocystis infection is thought to be maintained in host populations by airborne circulation, as the organism is capable of at least transiently colonizing
and replicating in the lung of immunocompetent hosts, and can be spread between healthy hosts as well as to immunocompromised susceptible hosts.(1) In the lung,
life cycle stages recognized include a 1-4 micron diameter, thin-walled vegetative or trophic form, which attaches to type 1 alveolar epithelial cells and appears
to develop through three consecutive sporocyte stages to produce a 3-8 micron diameter, thick-walled cyst form, in which multiple nuclear divisions lead to the
formation of eight intracystic spores.(1) These intracystic bodies are released and presumably develop into trophic forms. (Note that the names of these stages still reflect the original classification of these organisms as protozoa; appropriate renaming of these stages as fungal developmental stages awaits further elucidation of the sexual stages of the cycle). Binding to type I pneumocytes as well as to macrophages is mediated by the cell wall major surface glycoprotein A. Both macrophages and cell-mediated immunity are
important for control of infection, and development of clinical disease implies impairment of either macrophage function or cell-mediated immunity.(2)
The ferret has been used as an animal model of Pneumocystis pneumonia induced by corticosteroid administration, while development of disease in pet ferrets is recognized as a potential complication of long-termcorticosteroid therapy or hyperadrenocorticism.(3) In this case, no predisposing condition was identified. Both adrenal glands were
histologically unremarkable (although the right adrenal had a small intracortical cyst). Extrapulmonary localization of the organisms was apparent in this case,
and has been reported in liver and kidney from 10% of experimentally immunosuppressed ferrets.(6)
JPC Diagnosis:
Lung: Pneumonia, interstitial, histiocytic and necrotizing, diffuse, marked, with numerous intra-alveolar fungal cysts consistent with
Pneumocystis carinii.
Conference Comment:
The contributor provided a good summary of disease associated with
Pneumocystis species. As stated,
Pneumocystis can affect several species of animals, usually causing disease only in immunocompromised hosts. Interestingly, however, studies have linked
Pneumocystis carinii to infectious interstitial pneumonia (IIP), a common, transient, usually mild disease of immunocompetent laboratory rats that has been, until recently, attributed to an alleged virus referred to as rat respiratory virus (RRV). The distinctive lung lesions (lymphohistiocytic interstitial pneumonia with perivascular lymphocytic cuffs) associated with IIP, first described in the mid-1990s, led researchers to suspect a viral cause. Subsequent studies showed the presence of a virus that could be propagated in cell culture; this virus was referred to as RRV. However, the RRV antibody assays did not correspond to the IIP status of colonies as determined by histopathology, leaving histopathology as the only reliable diagnostic method for rat colony surveillance.
Since then, several investigations have demonstrated a cause and effect relationship between
Pneumocystis carinii and IIP in immunocompetent rat colonies using
PCR and IFA, as well as histopathology. These studies also found that the concentration of
Pneumocystis carinii in the lung corresponded with the histologic
severity of IIP. Furthermore, clearance of
Pneumocystis carinii lung infection via a humoral response corresponded with resolution of pneumonia.Â
Pneumocystis carinii is the most common
Pneumocystis species found in rats, occurring much more frequently than
Pneumocystis wakefieldiae. Pneumocystis wakefieldiae was not found to be associated with IIP.(4)
References:
1. Aliouat-Denis C-M, Chabe M, Demanche C, et al. Pneumocystis species, co-evolution and pathogenic power.Â
Infect Genet Evol. 2008;8:708-726.
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed.
Jubb, Kennedy, and Palmers Pathology of Domestic Animals.5th ed. Philadelphia, PA: Saunders Elsevier; 2007:593.
3. Dei-Cas E, Brun-Pascaud M, Bille-Hansen V, et al. Animal models of pneumocystosis.Â
FEMS Immunol Med Microbiol. 1998;22:163-168.
4. Henderson KS, et al. Pneumocystis carinii causes a distinctive interstitial pneumonia in immunocompetent laboratory rats that had been attributed to rat respiratory virus.Â
Vet Pathol. 2012;49: 440-452.
5. Oz HS, Hughes WT, Vargas SL. Search for extrapulmonary Pneumocystis carinii in an animal model.Â
J Parasitol. 1996;82:357-359.
6. Wakefield AE. Genetic heterogeneity in Pneumocystis carinii: an introduction. FEMS
Immunol Med Microbiol. 1998;22:5-13.