Signalment:
6-month-old, male, domestic ferret (
Mustela putorius furo)This 6-month-old, male domestic ferret
presented to the referring veterinarian with a 1-2 week
history of diarrhea and lethargy. On physical exam, the
animal was assessed as being moderately dehydrated. The
abdomen was moderately distended. Abdominal ultrasound
showed marked enlargement of the spleen, multifocal areas
of hyperechogenicity in the liver, and markedly enlarged
mesenteric lymph nodes. Over the next week, diarrhea
continued and the ferret became anorexic, lost weight, and
developed a mild cough despite symptomatic treatment.
Euthanasia was elected and the animal was submitted to
the Diagnostic Center for Population and Animal Health,
Lansing, MI for necropsy.
Gross Description:
On gross necropsy, the animal was
in fair to poor body condition with no visible fat stores and
increased prominence of bony protuberances. Dehydration
was marked as evinced by retraction of the eyes into the
orbits and tackiness of visceral surfaces. The abdomen
was prominently distended due to marked enlargement
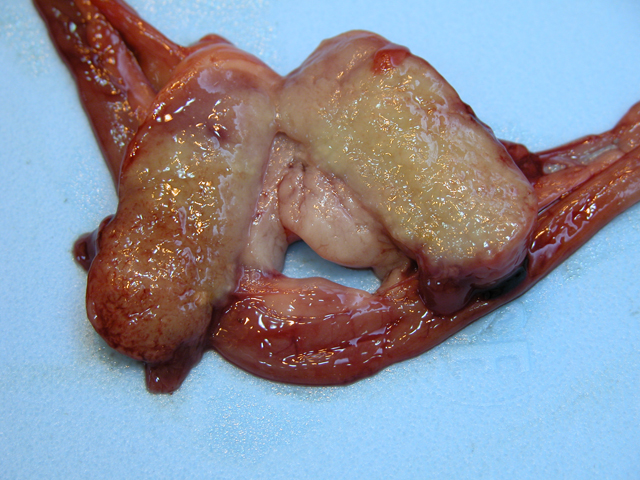
of the spleen and mesenteric lymph nodes. The spleen
was approximately 10 times normal size, dark black and
diffusely meaty. There were 7-8 relatively indistinct,
poorly circumscribed, pale white, semi-firm, nodular foci
dispersed throughout the splenic parenchyma. These foci
ranged from 0.5-1.5 cm in maximum diameter, and the
capsular surface of the spleen overlying few of these areas
was slightly raised. The liver was diffusely mottled dark
red and tan, and was mildly enlarged with slightly rounded
borders. There were 10-12 slightly raised, occasionally
coalescing, pale white, firm, slightly nodular plaques
ranging from 0.5-1 cm in diameter randomly distributed
on the capsular surface of the liver. The mesenteric lymph
nodes were markedly enlarged being approximately 5-8
times normal size. The capsular surfaces of lymph nodes
were irregular due to dozens of 2-5 mm in diameter,
slightly raised white nodules. On cut surface, the normal
nodal architecture was obliterated by pale tan to white, firm
tissue (
Fig. 3-1). The mesentery was irregularly thickened
by dozens of coalescing pale white, firm nodules and
irregular plaques. Similar white nodules and plaques were
segmentally distributed across the serosal surfaces of the
jejunum, ileum and to a lesser degree colon. In few areas,
sections of intestinal loops were adhered to each other
and to adjacent mesenteric lymph nodes by both fibrinous
and fibrous adhesions. The visceral pleural surfaces of
the lung were focally raised 1-3 mm by similar irregularly
shaped, pale white, poorly demarcated, firm plaques that
ranged from .2-1.5 cm in diameter. No other significant
lesions were noted grossly.
Histopathologic Description:
In the wall of the small intestine, there are multifocal to
coalescing areas of granulomatous to pyogranulomatous
inflammation. These areas are most prominent on the
serosal surfaces of the intestine, but also extend into the
underlying tunic muscularis and more rarely into the
submucosa and mucosa. Granulomas and pyogranulomas
are characterized by variable central necrosis and
infiltrates of degenerate neutrophils surrounded by
thick dense bands of plump epithelioid macrophages
and rare multinucleated foreign body type giant cells.
Varying rings of lymphocytes, plasma cells, and dense
maturing fibrosis surround granulomas. Granulomatous
inflammation is generally randomly distributed, but
occasionally is associated with vasculature. In sections of
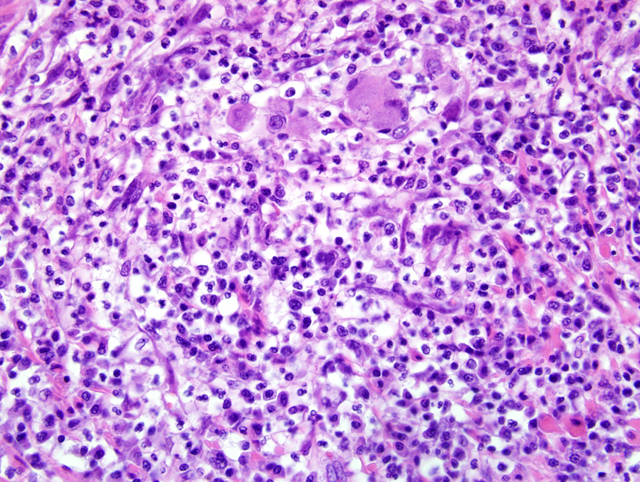
lymph node, nodal architecture is expanded and ablated by
multifocal and coalescing granulomas and pyogranulomas
similar to those described in the small intestine (
Fig. 3-2).
Granulomatous inflammation extensively extends through
the capsule into the surrounding perinodal adipose
tissue. Similar foci of granulomatous to occasionally
pyogranulomatous inflammation expand the serosal
surfaces of the liver and lung, and focally obliterate the
normal parenchyma of the lung and spleen. In rare areas,
the meninges overlying the cerebral cortex are expanded
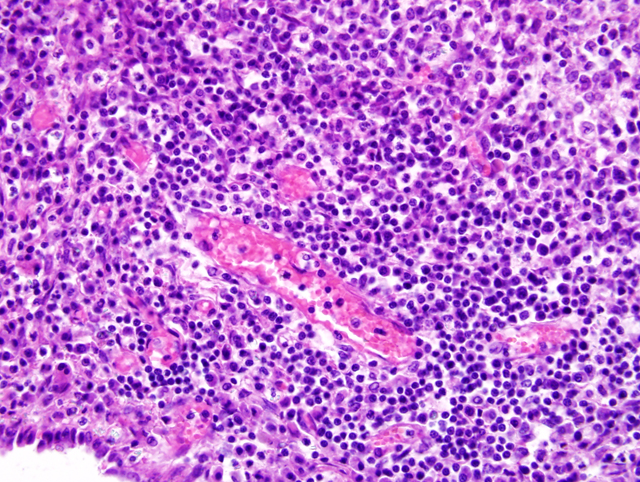
by granulomatous inflammation. Vasculature within tissue
surrounding foci of granulomatous inflammation in the
liver, lung, and cerebral cortex is segmentally surrounded
by moderate cuffs of lymphocytes, plasma cells, and
fewer histiocytes (
Fig. 3-3). Immunohistochemistry
using a generic antibody against Group 1 coronaviruses
on sections of small intestine, lymph node, lung, and
spleen demonstrated strong positive, intracytoplasmic
immunoreactivity within macrophages at the center of
granulomas. A generic coronavirus RT-PCR yielded
amplification of a 650 base pair fragment. Sequencing
of this segment suggests that the amplified virus is
distinct from feline coronavirus (FeCoV). The amplified
virus appears to be most closely related to ferret enteric
coronavirus (FECV) that reportedly causes epizootic
catarrhal enteritis (ECE) in ferrets.
Morphologic Diagnosis:
Small
intestine: Severe chronic segmental granulomatous to
pyogranulomatous enteritis and peritonitis
Lymph node: Severe chronic multifocal and coalescing
granulomatous to pyogranulomatous lymphadenitis
Lab Results:
PCR for Aleutian mink disease virus was negative.
Condition:
Coronavirus-associated granulomatous disease
Contributor Comment:
This case presentation
represents a disease that has been termed granulomatous
inflammatory syndrome (GIS) or ferret systemic
coronavirus infection (FSCV).1,4 This is an emerging
disease in ferrets that was first reported in the veterinary
literature in 2006 and closely resembles the clinical,
gross and microscopic features of feline infectious
peritonitis.
1,2,4
The exact etiopathogenesis of this lesion is unclear at this
point, but appears to be related to infection with a group
1 coronavirus. Immunohistochemistry using monoclonal
antibodies against feline coronavirus (FCoV) has been
reported to demonstrate immunoreactivity in macrophages
within lesions.
1,2 This antibody is not specific for FCoV
as it has been shown to detect other group 1 coronaviruses
including ferret enteric coronavirus (FECV) that has been
implicated as the cause of epizootic catarrhal enteritis
(ECE).
5,6 Electron microscopy confirmed the presence
of coronavirus-like particles within macrophages.
1 RTPCR
using primers that detect a broad array of group
1 coronaviruses yielded amplification of a 599bp
sequence that showed significant similarity to FECV
(77% homology) and to other group 1 coronaviruses.
1
Whether this virus represents a novel virus or variation
within an already described coronavirus is unclear. The
exact mechanism by which this virus causes the described
lesions is unknown. Further characterization of the virus
is required.
JPC Diagnosis:
Small intestine: Enteritis and peritonitis, pyogranulomatous, multifocal to coalescing, moderate
Lymph node: Lymphadenitis, pyogranulomatous, multifocal to coalescing, moderate
Conference Comment:
Coronaviruses are singlestranded
RNA viruses of major importance in domestic
animals.
3 Coronaviruses are currently split into 3
serogroups. Group 1 includes transmissible gastroenteritis
of swine (TGEV), canine coronavirus (CCV), feline
coronavirus (FCoV), and human coronavirus 229E.
Mouse hepatitis virus, sialodacryoadenitis of rats, turkey
coronavirus (bluecomb), and bovine coronavirus are the
major viruses recognized in group 2.
1,3 Group 3 comprises
the avian viruses and includes infectious bronchitis virus.
1,3
As the contributor mentioned, ferret systemic coronavirus
infection (FSCV) has an almost identical gross and
histologic appearance as FIP in domestic cats. Juvenile and
young adult ferrets seem to be the most susceptible to this
disease.(1) Gross lesions consist of widespread nodular
foci on multiple serosal surfaces that closely resemble
similar foci seen in clinical cases of FIP.
1 Involvement of
mesenteric lymph nodes is also reminiscent of FIP in cats.
FSCV closely resembles the dry form of FIP.
1 Histologic
lesions are identical to what is seen in cats with FIP and
consist of pyogranulomatous inflammation with vasculitis
and perivasculitis.
1 Relevant clinical pathologic findings
in these ferrets included a mild non-regenerative anemia
(anemia of chronic disease), thrombocytopenia and
hyperproteinemia.
1 The thrombocytopenia was attributed
to DIC secondary to vasculitis, while the hyperproteinemia
occurred due to hyperglobulinemia.
1
References:
1. Garner MM, Ramsell K, Morera N, Juan-Sall+�-�s C,
Jim+�-�nez J, Ardiaca M, Montesinos A, Teifke JP, L+�-�hr
CV, Evermann JF, Baszler TV, Nordhausen RW, Wise
AG, Maes RK, Kiupel M. Clinicopathologic features of
a systemic coronavirus-associated disease resembling
feline infectious peritonitis in the domestic ferret (
Mustela
putorius). Vet Pathol
45(2):236-46, 2008
2. Mart+�-�nez J, Ramis AJ, Reinacher M, Perpi+�-�+�-�n D.
Detection of feline infectious peritonitis virus-like antigen
in ferrets. Vet Rec
158:523, 2006
3. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ:
Coronaviridae.Â
In: Veterinary Virology, 3rd ed., pp. 495-
508. Academic Press, San Diego, California, 1999
4. Perpi+�-�+�-�n D, L³pez C. Clinical aspects of systemic
granulomatous inflammatory syndrome in ferrets (
Mustela
putorius furo). Vet Rec
162(6):180-185, 2008
5. Williams B, Kiupel M, West K, Raymond JT, Grant
CK, Glickmann LT, Coronavirus associated enzootic
catarrhal enteritis (ECE) in ferrets (
Mustela putorius furo):
a review of 120 cases (1993-1998) JAVMA 217: 526-530,
2000
6. Wise AG, Kiupel M, Maes RK. A novel coronavirus
associated with epizootic catarrhal enteritis (ECE) in
ferrets. Virol
349:164-174, 2006