WSC 2023-2024, Conference 18, Case 2
Signalment:
Adult male striped skunk (Mephitis mephitis)
History:
This striped skunk was found alive but twitching in a residential area of Charlottetown, Prince Edward Island. The skunk was euthanized and submitted for post mortem examination.
Gross Pathology:
The skunk was in good body condition. The caudodorsal lung lobes were bright red and slightly firm with numerous flat, white, 2 mm in greatest diameter, white foci randomly scattered throughout all lung lobes. The trachea and mainstem bronchi contained pink-tinged froth. The liver was diffusely and mildly enlarged, dark red and friable and all liver lobes contained numerous pinpoint, white, flat, round foci. There were multiple skull fractures with clotted blood multifocally within the temporal muscles and meninges.
Laboratory Results:
Real-time RT-PCR for Influenza A matrix gene on oropharyngeal swab: Positive.
Real-time RT-PCR for Avian Influenza Virus H5 subtype on oropharyngeal swab: Positive.
Microscopic Description:
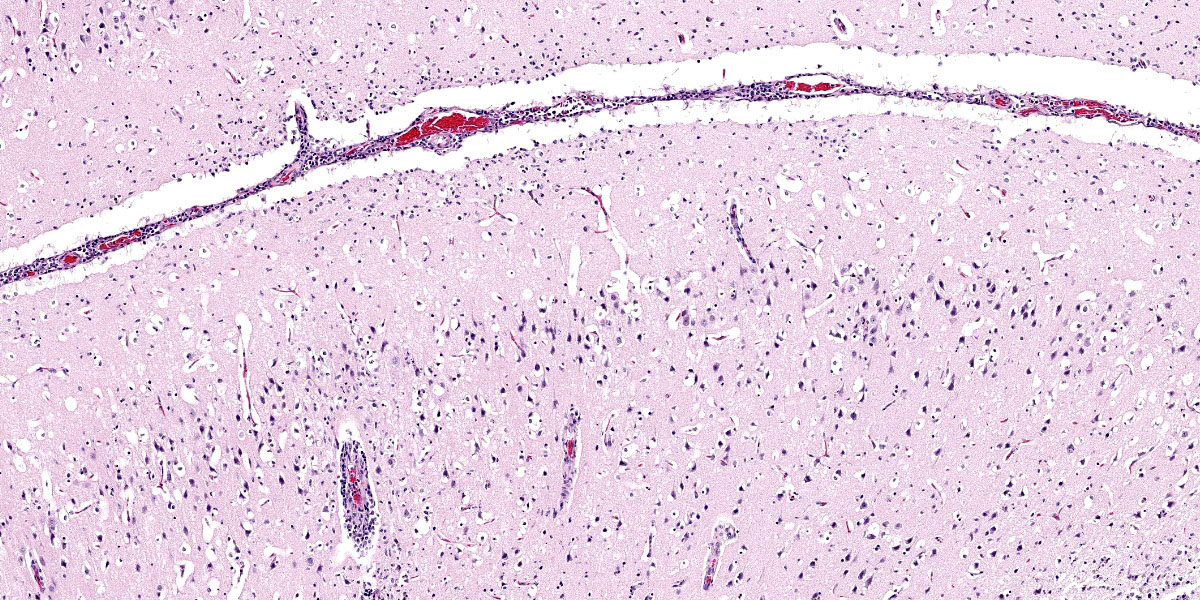
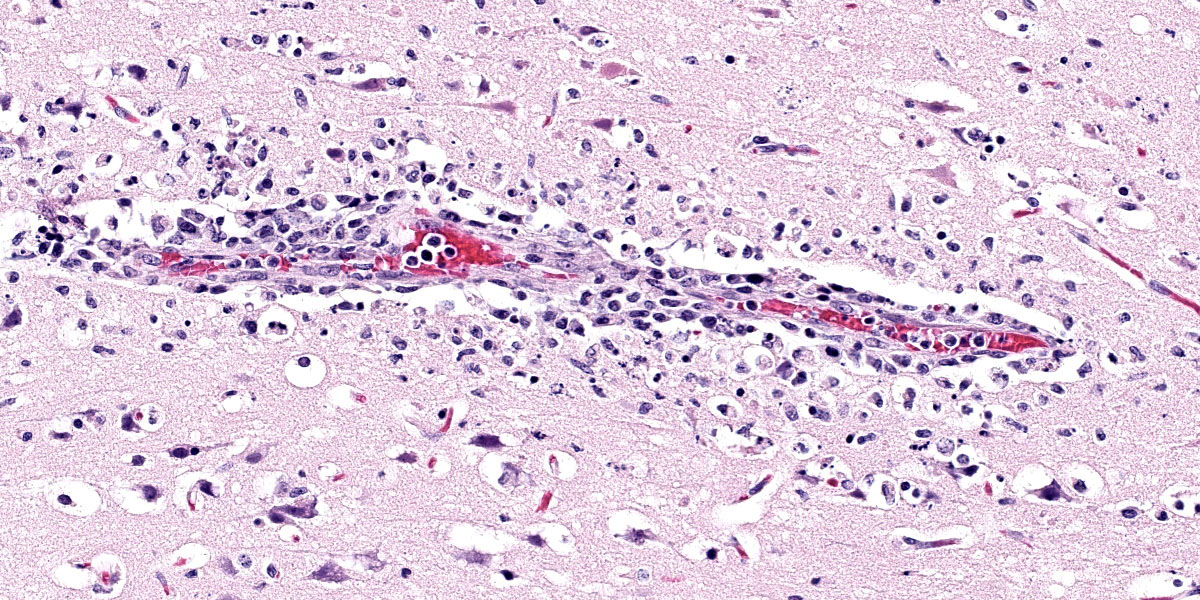
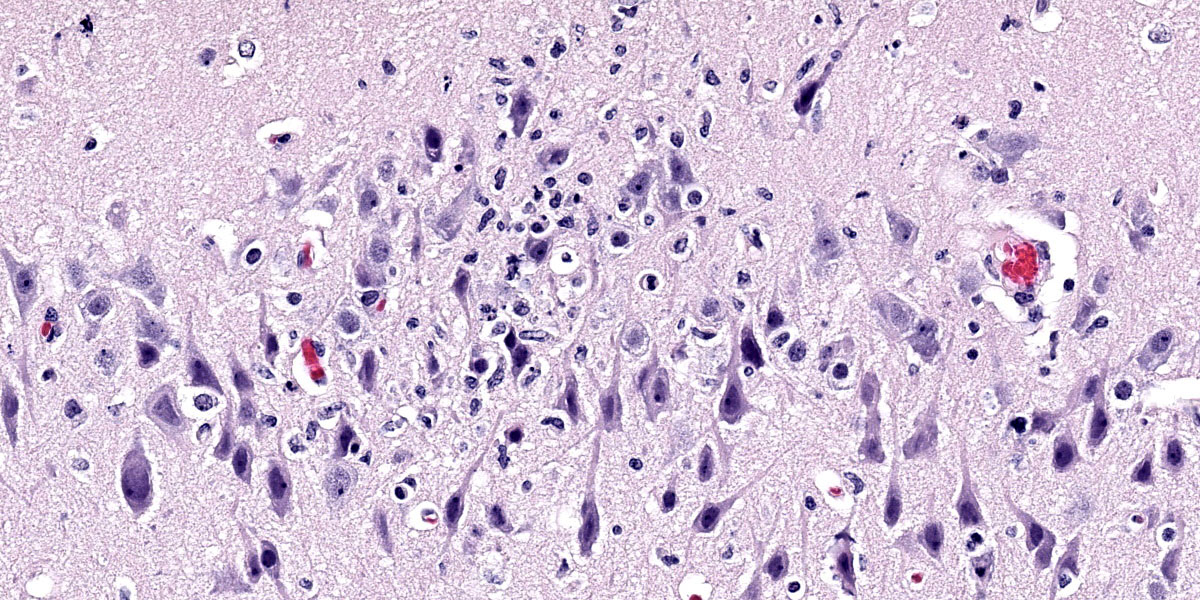
Brain: Surrounding numerous blood vessels in the cerebral grey matter and meninges are small numbers of degenerate neutrophils, lymphocytes, plasma cells, microglial cells and pyknotic cellular debris. Adjacent to these areas in the cerebral cortex, neurons are occasionally hypereosinophilic and shrunken with pyknotic nuclei (acute degeneration/necrosis) and are surrounded by small numbers of microglial cells. The Virchow Robin space is diffusely expanded by small to moderate numbers of lymphocytes and plasma cells. The grey matter neuropil is moderately hypercellular with an increased number of microglial and glial cells. Meningeal blood vessels are diffusely cuffed by lymphocytes and plasma cells and there are multiple areas where the meninges contain moderate numbers of erythrocytes (hemorrhage).
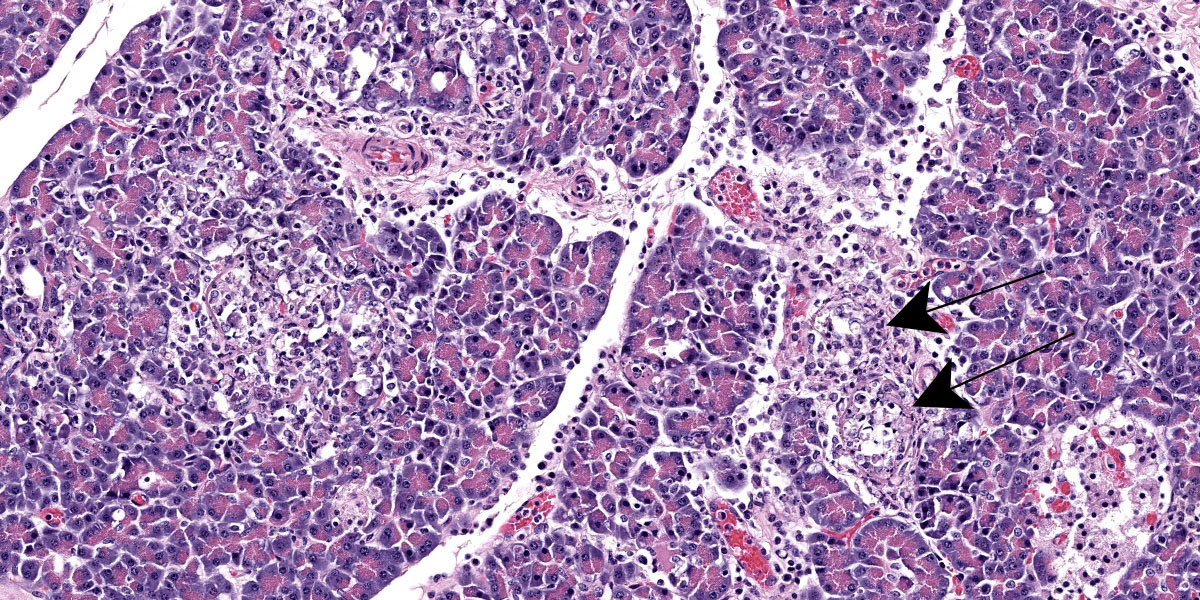
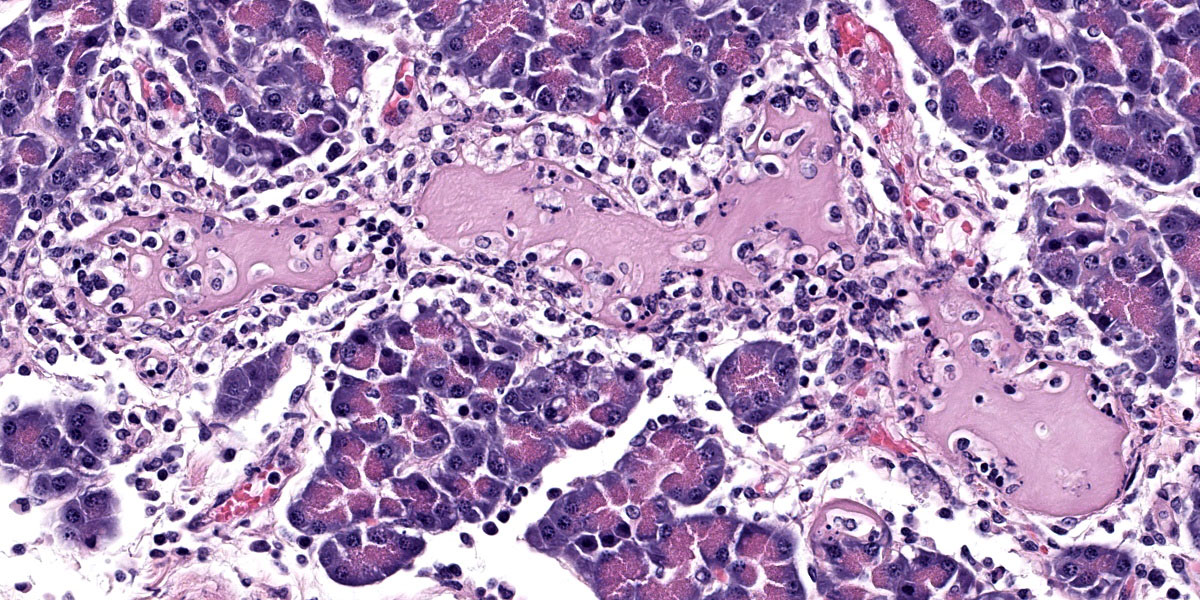
Pancreas: Randomly scattered throughout the exocrine pancreas, replacing pancreatic acini and occasionally surrounding blood vessels are numerous, variably-sized nodular aggregates that are composed of moderate numbers of degenerate neutrophils, macrophages, eosinophilic granular material, and pyknotic cellular debris . These areas often contain moderate amounts of pale eosinophilic, amorphous, fibrillar to flocculent, extracellular matrix (amyloid). Pancreatic acini in the periphery of these areas are often fragmented with singular acinar cells remaining. The interstitium contains moderate numbers of neutrophils, macrophages, eosinophilic fluid, and fewer lymphocytes and plasma cells and pyknotic cellular debris. The lumen of pancreatic ducts often contains small numbers of neutrophils
Contributor’s Morphologic Diagnosis:
1. Brain: Necrotizing meningoencephalitis, multifocal, acute, severe, with multifocal acute neuronal necrosis, gliosis and satellitosis
2. Brain: Multifocal, acute, severe, meningeal hemorrhage
3. Pancreas: Necrotizing pancreatitis, multifocal, subacute, severe with multifocal acinar loss
Contributor’s Comment:
Influenza viruses are enveloped RNA viruses that have the potential to undergo gene reassortment in a host when they are infected with multiple viruses.1,3 Gene reassortment can lead to the development of highly pathogenic strains that infect multiple species and can result in pandemics.3
The A/Goose/Guangdong/1/96 lineage of H5 highly pathogenic avian influenza virus (HPAI), which first emerged in Southeast Asia in 1996, was detected in wild and domestic birds in Atlantic Canada in late 2021.2 H5N1 viruses in Newfoundland, Canada, in late 2021 were most closely related to HPAI viruses circulating in northwestern Europe in spring 2021, and were most likely carried across the Atlantic via migratory birds.2 Since that time, H5N1 viruses have spread throughout North America, resulting in neurologic signs and widespread mortality in a wide variety of wild birds and mammals, as well as significant impacts on the poultry industry.1 In early 2023, fully Eurasian H5N5 viruses began to be detected in wild birds in Atlantic Canada, presumably representing a new incursion to the region via migratory birds. Genetic analysis of samples from the skunk in this submission revealed H5N5 subtype of the 2.3.4.4b Eurasian lineage of HPAI.
In Atlantic Canada, H5N1 HPAI has been detected in red foxes (Vulpes vulpes), striped skunks (Mephitis mephitis), and raccoons (Procyon lotor), with additional diagnoses in these species and other mammals throughout North America.1 H5N5 HPAI has been detected in raccoons, one striped skunk, and multiple American crows in the region. Mammals infected with H5N1 HPAI typically show neurologic signs, including seizures, twitching, foaming at the mouth, circling, or are found dead.1 Gross post mortem lesions include pulmonary edema, bronchointerstitial pneumonia, nasal turbinate edema, multifocal (2 to 3 mm in greatest diameter) areas of necrosis throughout the pulmonary and hepatic parenchyma, and meningeal congestion. The most common microscopic lesions include meningoencephalitis, gliosis, bronchointerstitial pneumonia, pulmonary edema and congestion, necrotizing hepatitis and cerebral and brainstem neuronal necrosis.1 Although the lesions in H5N5 HPAI positive cases are not yet described, the gross and microscopic lesions in this case are consistent with those described in H5N1 HPAI positive skunks and other North American mammals in the current outbreak.
Contributing Institution:
Atlantic Veterinary College, University of Prince Edward Island
Department of Pathology and Microbiology
https://www.upei.ca/avc/pathology-and-microbiology
JPC Diagnosis:
1. Cerebrum: Meningoencephalitis, necrotiing and lymphohistiocytic, diffuse, mild to moderate.
2. Pancreas: Pancreatitis, necrotizing and lymphoplasmacytic, diffuse, moderate.
3. Pancreas: Amyloidosis, multifocal, mild.
JPC Comment:
As noted by the contributor, Influenza A viruses exchange genetic information through reassortment of their segmented RNA genomes. Reassortment, a method to supercharge viral evolution, is a strategy available only to RNA viruses with segemented genomes and requires concurrent co-infection with more than one Influenza A strain. Reassortment occurs when RNA segments from different influenza viruses are intermixed during the virion assembly process, resulting in a large genomic change and the development of a new virus subtype, a process known as genetic shift. This is in contrast to the emergence of new virus types due to random point mutations, a slower, more conservative change known as antigenic drift.
Influenza A viruses have a broad host range, with established lineages circulating in poultry, pigs, humans, and other mammals, and a particularly rich tapestry of viral diversity circulating in wild birds.3 Particular influenza viral lineages tend to be restricted to their preferred mammalian hosts; however, reassortment occasionally produces novel influenza viruses that are able to cross species barriers and cause viral pandemics.3 Research has shown that such reassortment occurs frequently in vivo, leading to viral genotypic diversity both between animals and among the various levels of the respiratory tract in individual animals.3
Pigs are of particular interest in influenza ecology as they are susceptible to swine, human, and avian influenzas, and thus provide an accommodating microbial mixing vessel that can give rise to recombinant viruses.3 These novel viral subtypes have been behind most major historical influenza pandemics to date, and the emergence of a new viral influenza subtype with pandemic potential is an ever-present threat.4
This case generated robust discussion, particulary around changes within the neuroparenchyma. Multiple conference participants were convinced that many neurons contained intranuclear eosinophilic viral inclusions, which would not be expected with influenza infection. The discussion quickly turned to the age-old “inclusion body or nucleoli” debate without satisfactory resolution. Several participants also believed there to be neuronal loss, though while neuronal necrosis was evident, most participants felt that the substantial gliosis made the neuroparenchyma appeared hypercellular, not hypocellular.
Despite the nuances in the neuroparenchymal pathology, the moderator emphasized that the nature and distribution of the inflammation in section are highly suggestive of a viral etiology. Participants discussed various differential diagnoses before noting that the presence of necrotizing pancreatitis considerably narrowed the differential list. Differentials for pancreatic necrosis in a bird include West Nile Virus, Avian Adenovirus, HPAI, Sarcocystis sp., and zinc toxicity; however, the most likely cause of necrotizing pancreatitis and meningoencephalitis in a bird is HPAI.
The ensuring morphologic diagnosis discussion was scentillating. Participants were skeptical that the meningeal hemorrhage was replated to the HPAI and wondered if this could be euthanasia artifact. Participants were similarly unconvinced that the amyloid noted in the pancreatic acini and islets was due to HPAI infection. The agreed-upon morphologic diagnosis highlights the necrosis that characterizes the disease and separates out the amyloidosis as a discrete disease process.
References:
- Alkie TN, Cox, S, Embury-Hyatt C, et al. Characteriziation of neurotropic HPAI H5N1 viruses with novel genome constellations and mammalian adaptive mutations in free-living mesocarnivores in Canada. Emerg Microbes Infect. 2023;12(1):2186608.
- Caliendo V, Lewis NS, Pohlmann A, et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci Rep. 2022;12(1):1-18.
- Ganti K, Bagga A, Caranccini S, et al. Influenza A virus reassortment in mammals gives rise to genetically distinct within-host subpopulations. Nat Commun. 2022;13(1): 6846.
- Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning S, Hartigan PJ. Veterinary Microbiology and Microbial Diseases. Blackwell Publishing Ltd;2011: 647-659.