Signalment:
Gross Description:
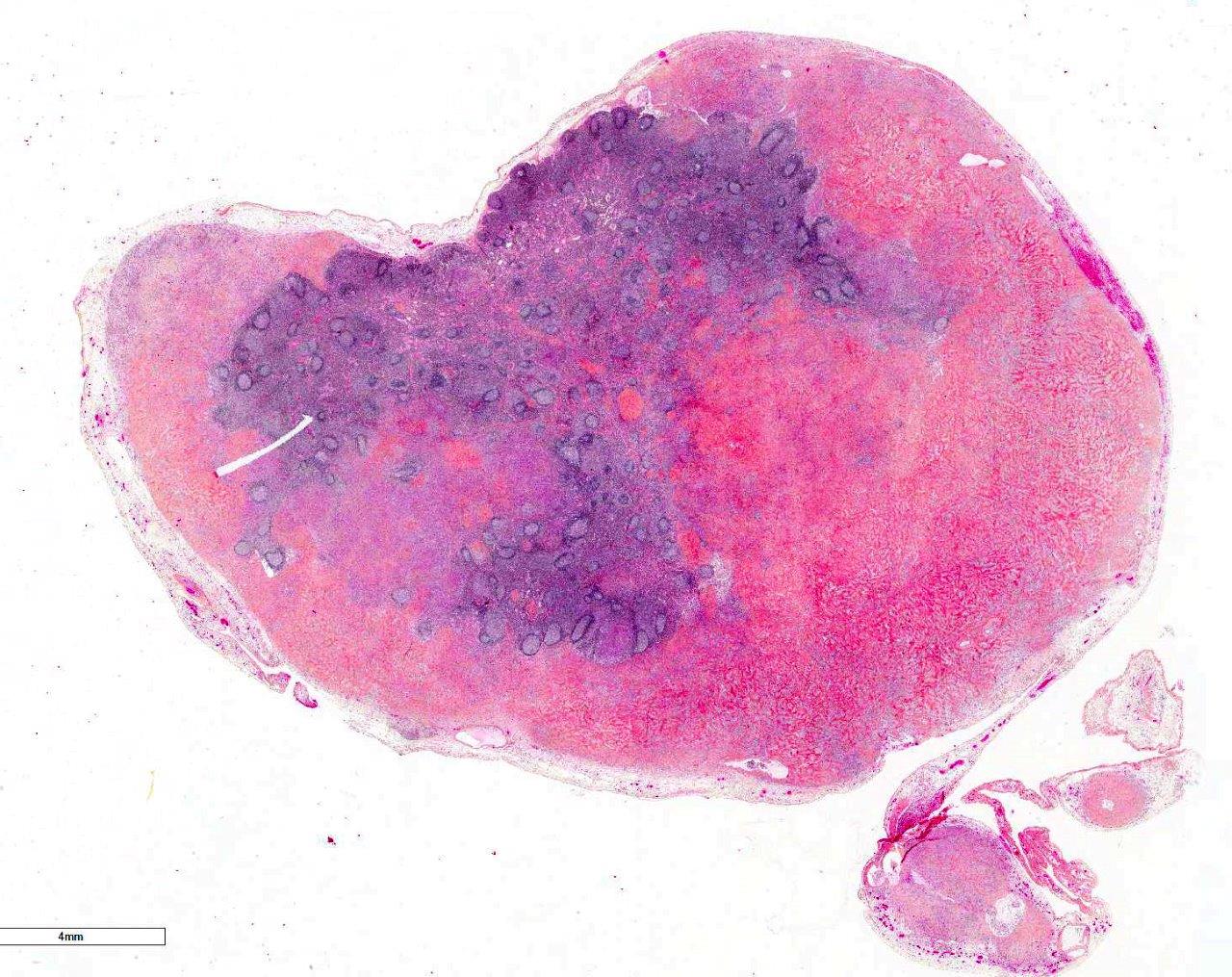
Histopathologic Description:
Intestinal wall/mass (not submitted): Lesions similar to the mesenteric lymph nodes diffusely efface and replace the ileocecocolic submucosa and tunica muscularis, focally creating a mass effect. The mucosa has markedly increased numbers of eosinophils (lamina propria), and is multifocally and extensively ulcerated with granulation tissue. The lumen contains fibrinonecrotic debris admixed with numerous bacteria.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
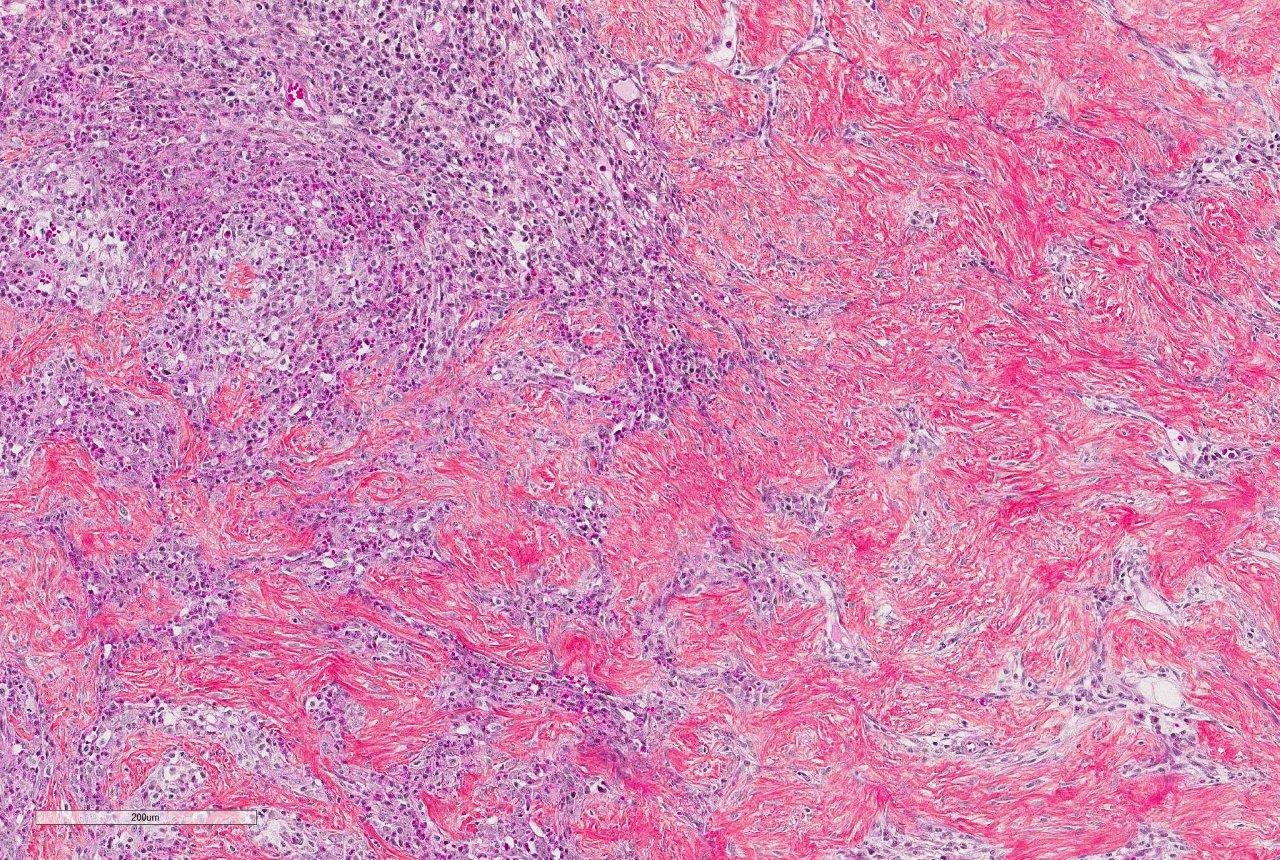
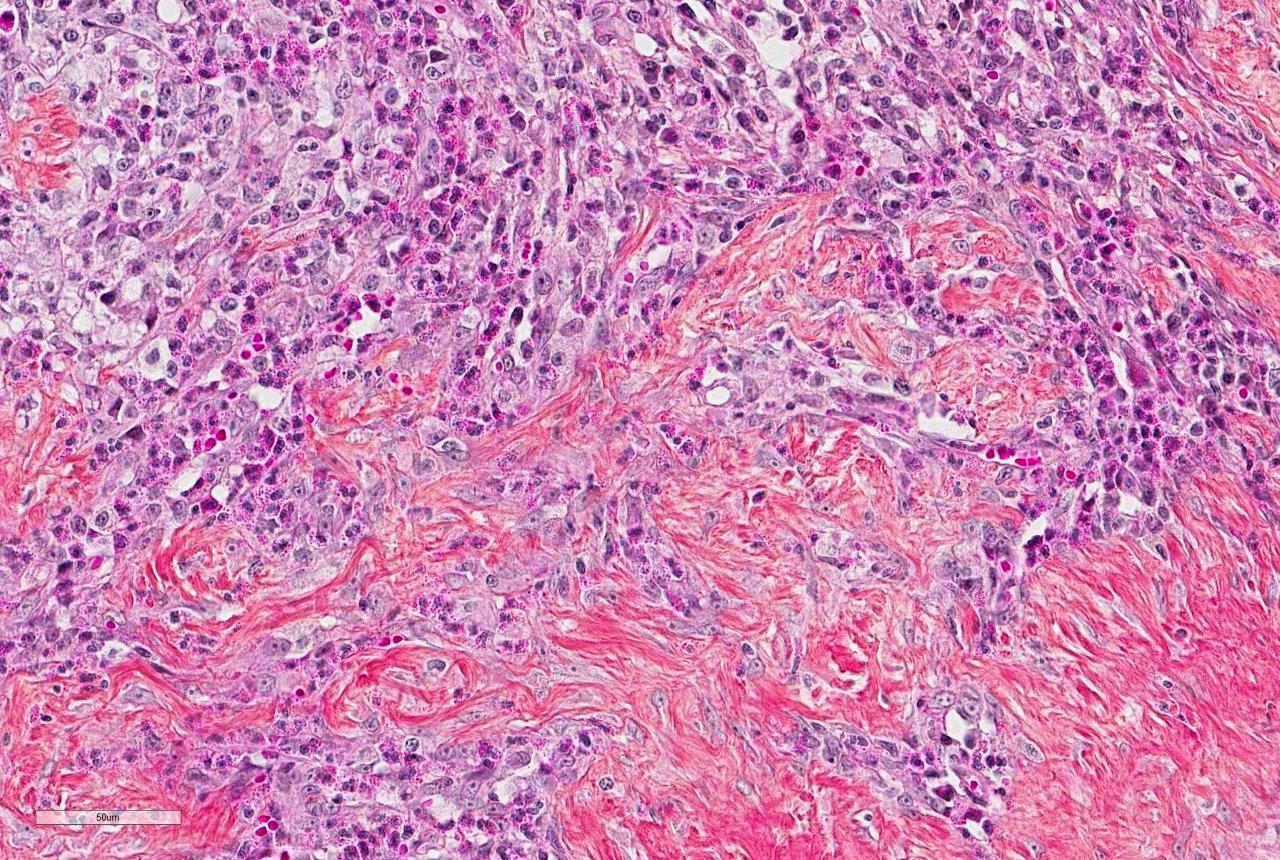
Histologically, FGESF lesions exhibit a characteristic pattern made of a network of coarse collagen trabeculae admixed with a large spindle cell population identified as myofibroblasts1 mixed with inflammatory cells including numerous eosinophils.1,5,7,8 The fibroplasia and inflammatory response extensively infiltrate the intestinal wall and, sometimes, regional lymph nodes. The intestinal mucosa is frequently ulcerated. Grossly and histologically, FGESF may resemble neoplasia, particularly, fibro-sarcoma and extra-skeletal osteosarcoma; thus, FGESF must be included in the differential diagnosis of cats presented for abdominal mass and/or mesenteric lympha-denopathy.
The etiopathogenesis of this condition is still not completely understood. The presence of bacteria, including gram-positive cocci as well as gram-positive and gram-negative rods have been associated with lesions in several studies,1,5,8 but it is still unclear how bacteria contribute to the development and/or perpetuation of the condition. The location of lesions in close proximity or communicating with the normal bacterial flora makes it complicated to establish an association. In one study, IHC was performed and was negative for both FHV-1 and FCoV/FIP viral antigens.5 It was also hypothesized that affected cats could suffer from immunologic dysregulation triggered by one or few factors, including parasitism, food allergy, dysbiosis or certain forms of IBD but again, no clear associations have been made. Nevertheless, eosinophilic inflammation seems to be a critical feature of this condition and is consistently predominant. Eosinophils produce a variety of mediators, including major basic protein (MBP), TGF-β, Il-1b, IL-6, that lead to tissue destruction and fibrosis.1,5,7,8 A perpetuating inflammatory and fibrotic process is thought to be responsible for the proliferative nature of the pathology that gives rise to an intra-abdominal mass. Prognosis varies from good to guarded since infiltration is sometimes extensive and not surgically resectable (if located at the pyloroduodenal junction) but survival times can be good with a combination of appropriate surgical and medical treatments.1,5,8
JPC Diagnosis:
Conference Comment:
Prior to the initial description and publication of this unique lesion in the feline GI tract by Craig et al, the characteristic trabeculae of dense collagen were often misinterpreted as osteoid, leading to an erroneous diagnosis of extra-skeletal osteosarcoma.1 Additionally, the marked eosinophilic component admixed with scattered, well-differentiated, perivascular mast cells resulted in the interpretation of some of these lesions as feline sclerosing mast cell tumors.4 In this case, histochemical staining with Massons trichrome produced intense blue staining of the anastomosing trabeculae, confirming the presence of collagen.
Although the etiology and pathogenesis of this lesion is unknown, the conference moderator (Dr. Craig, referenced above) posits that it is likely an inflammatory process rather than neoplastic, similar to other feline eosinophilic inflammatory lesions, such as feline indolent ulcer, eosinophilic plaque, eosinophilic granuloma, and hypereosinophilic syndrome.1,3 As mentioned by the contributor, the majority of previously reported cases are associated with bacterial infection, demonstrated by both gram-positive and gram-negative rods and cocci embedded within the sclerotic collagen;1 however, there have been reports of FGESF associated with fungal infection, Toxoplasma gondii 1 and the alimentary nematode parasite (Cylcoospirura spp.) in a free-ranging puma. Additionally, there have also been reports of similar lesions present in the subcutis and abdomen of cats in Japan caused by methicillin-resistant Staphy-lococcus spp.6
Interestingly, in nearly half of reported cases by Craig, et al, no infectious etiology was detected.1 In this case, a battery of histochemical stains run by the Joint Pathology Center prior to the conference failed to reveal any infectious agents within dense bands of collagen. In cats that develop eosinophilic granulomatous disease, there may be an inherited genetic mutation leading to eosinophil dysregulation and inappropriate eosinophilic response to a variety of inflammatory stimuli.1,3,7 This may contribute to the pathogenesis of this disease in certain predisposed individuals with or without evidence of an infectious agent.1
References:
1. Craig L, Hardam E, Hertzke D, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol. 2009; 46: 6370.
2. Eckstrand CD, Barr BC, et al. Nematode-associated intramural alimentary nodules in pumas are histologically similar to gastro-intestinal eosinophilic sclerosing fibroplasia in domestic cats. J Comp Pathol. 2013; 148(4):405-409.
3. Grau-Roma L, Galindo-Cardiel I, et al. A case of feline gastro-intestinal eosinophilic sclerosing fibroplasia associated with phycomycetes. J Comp Pathol. 2014; 141:318-321.
4. Halsey CHC, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997-2008). Vet Comp Oncol. 2010; 8:72-79.
5. Linton M, Nimmo J S, Norris J M, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J Feline Med Surg. 2015; 17:392-404.
6. Ozaki K, Yamagami T, Nomura K, Haritani M, Tsutsumi Y, Narama I. Abscess-forming inflammatory granulation tissue with gram-positive cocci and prominent eosinophil infiltration in cats: Possible infection of methicillin-resistant Staphylococcus. Vet Pathol. 2003; 40:283287.
7. Suzuki M, Onchi M and Ozaki M. A case of feline gastro-intestinal eosinophilic sclerosing fibroplasia. J Toxicol Pathol. 2013; 26:51-53.
8. Weissman A, Penninck D, Webster C, et al. Ultrasonographic and clinico-pathological features of feline gastrointestinal eosinophilic sclerosing fibroplasia in four cats. J Feline Med Surg. 2013; 15:148-154.