Signalment:
Gross Description:
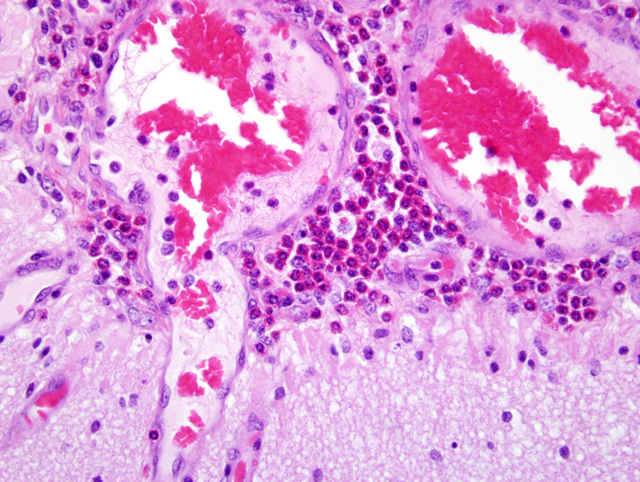
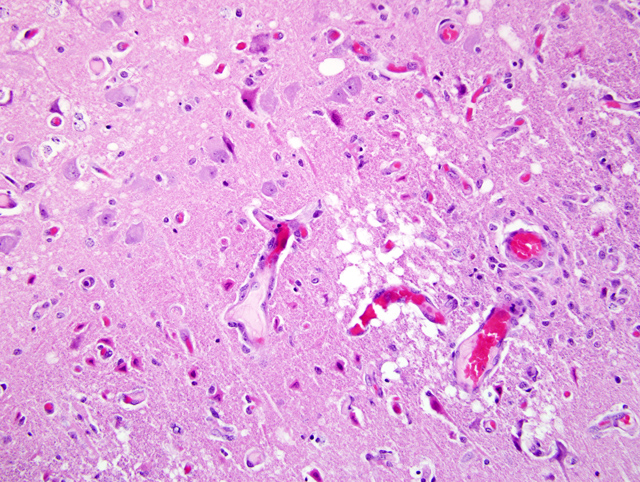
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In domestic animals direct salt poisoning occurs in cattle, horses, sheep and pigs with the ingestion of excessive amounts of sodium chloride. Direct salt poisoning can occur in pigs where bore water is used for livestock drinking or diets with excessive salt including salted fish waste, salt whey from cheese factories and excessive salt in baker's bread dough.(2) Indirect salt poisoning involves a normal intake of sodium chloride, but with limited water intake.(4) The latter is also termed water deprivation or water intoxication. Indirect salt poisoning has only been proven to occur in pigs, with circumstantial evidence in cattle and sheep.(1) Pigs are particularly susceptible to indirect salt poisoning because of the relatively high salt diets. Restriction of water intake to pigs, 4 to 12 weeks of age, fed prepared diets containing 2% salt can result in clinical disease.(2) Poisoning occurs when the animals again have access to unlimited water.
Indirect salt poisoning with decreased water intake, but normal sodium chloride intake results in an accumulation of sodium ions in the brain and other tissues over several days. High sodium accumulation inhibits anaerobic glycolysis, preventing active transport of sodium out of the cerebrospinal fluid. When there is access to water again, water migrates to the tissues to re-establish the salt-water balance. Acute cerebral edema develops and increased intracranial pressure results. Specific to pigs and of diagnostic significance, is that there is an influx of eosinophils into the meninges.(2,4)
In cases of salt poisoning there are two characteristic changes.(4) The first is eosinophilia of the leptomeninges and Virchow-Robin spaces in the cerebral cortex. The second, develops with increased duration of the lesion, is cerebral cortical necrosis and with advanced lesions cavitating areas of malacia.(1) A mixture of both reactions is most common, but either alone may be encountered.(4)
The differential diagnosis for CNS signs in young pigs includes viral encephalomyelitis, Aujeszky's disease, edema disease, Streptococcal meningitis, Glasser's disease, toxicosis, nutritional deficiencies and Mulberry heart disease.(3) Meningeal and perivascular infiltrates can occur in the brains of pigs with leukomalacia of Mulberry heart disease and other causes of encephalitis.(1) However, the combination of laminar cortical cerebral necrosis and cerebral eosinophilia is pathognomonic for direct or indirect salt poisoning.(1) Histologically, eosinophilic meningoencephalitis can also occur with parasitic infections. In Australia parasitic encephalitis is rare. In dogs Angiostrongylus cantonensis occurs infrequently, but has not been observed in pigs.Â
The importance of this report is that direct or indirect salt poisoning cases often affect many animals and mortalities can be high. In cases of indirect salt poisoning veterinary pathologists should recognize that a restricted water intake is often the cause and this may be due to an underlying animal welfare issue. Veterinary pathologists should recognize that they play an important role in recognizing or confirming animal welfare associated diseases.
JPC Diagnosis:
Conference Comment:
Idiopathic eosinophilic meningoencephalitis has also been reported in dogs and one cat. Severe neurologic signs including recumbency and loss of consciousness have been reported with this syndrome. Eosinophilia and CSF pleocytosis with a predominance of eosinophils are common clinical pathologic findings. Grossly, the meninges often have a green tinge because of the eosinophilic inflammatory infiltrate. Histologic changes include eosinophilic and granulomatous meningitis of cerebrum and cerebellum with eosinophilic perivascular cuffing. In dogs, Golden Retrievers and Rottweilers are the most commonly affected breeds.(1)
In pigs, salt toxicity can cause laminar cortical necrosis. Several of the submitted slides did have very good examples of neuronal necrosis, while other slides had a dearth of necrotic neurons. Slides submitted for this case were from three different pigs, so that may be the reason for slide variation. In ruminants, differentials for laminar cortical necrosis include lead poisoning, salt toxicity, sulfur toxicity, hypoxia, and thiamine deficiency.(1)
References:
2. Radostits OM, Gay CC, Hinchcliff KW, Constable PD: Diseases associated with inorganic and farm chemicals. In Veterinary Medicine. A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th ed. pp 1824 - 1826, Saunders Elsevier, Edinburgh, Scotland, 2007
3. Straw BE, Dewey CE, Wilson MR: Differiential diagnosis of swine disease. In Diseases of Swine 8th ed. Straw BE D'Allaire S, Mengeling WL, Taylor DJ. pp. 62-65, Blackwell Science, London, UK, 1999
4. Summers BA, Cummings JF, de Lahunta A: Degenerative Diseases of the Central Nervous System. In Veterinary Neuropathology. pp254-255. Mosby, St Louis, MO, 1995