WSC 22-23
Conference 7
CASE II:
Signalment:
6-month old, male (intact), pygmy goat Capra aegagrus hircus
History:
The goat was euthanized following several days of diarrhea and a 24-hour period of 'mental inappropriateness.' No additional history was provided.
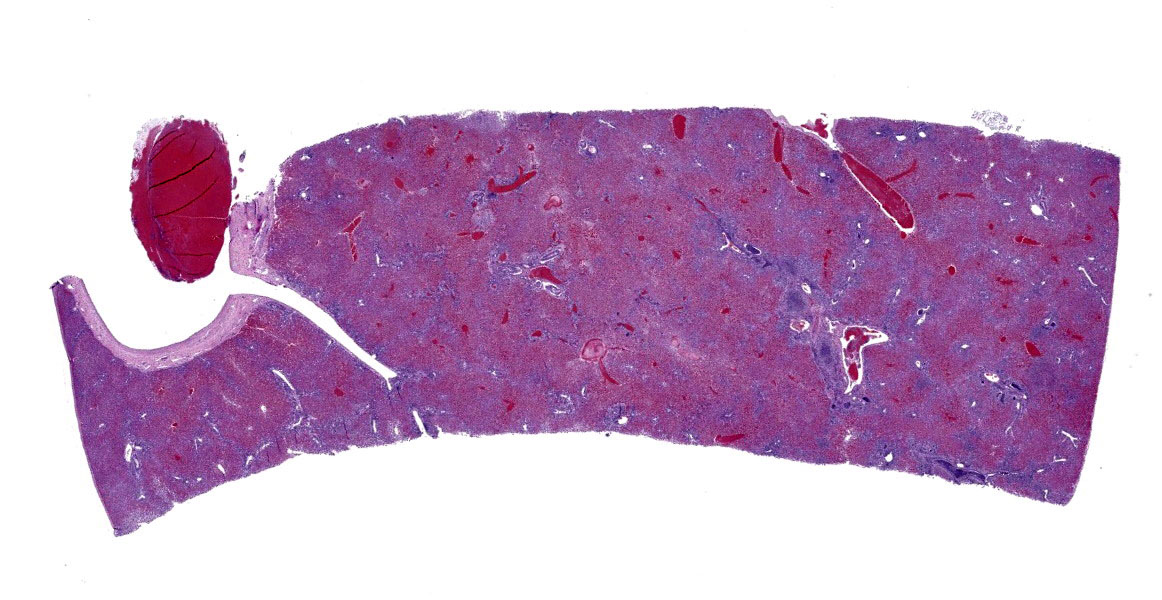
Gross Pathology:
The liver was enlarged, with 1 mm, soft, white, foci widely disseminated throughout capsular and cut surfaces. A focal, 1 cm in diameter ulcer was present in the abomasum near the pylorus, with adhered margins and subadjacent, firm, pale tan thickening of the wall (fibrosis).
Laboratory Results:
FA negative for rabies
FA positive, brain and liver, for Listeria monocytogenes
Aerobic culture, very light growth unidentified Gram-negative bacterium, enrichment broth culture negative for L. monocytogenes
Microscopic Description:
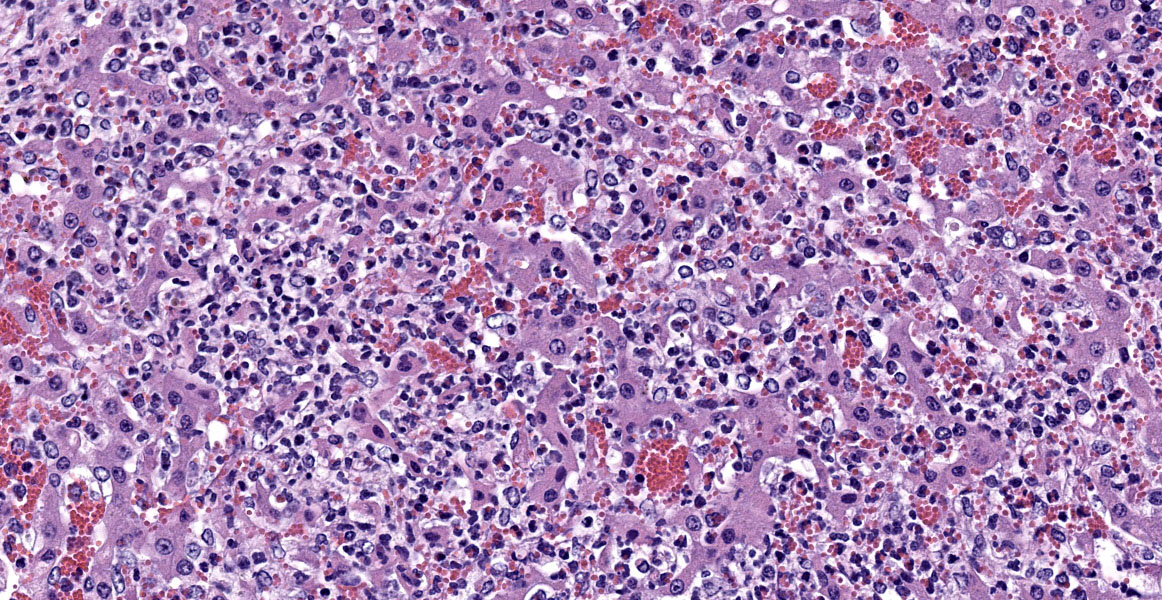
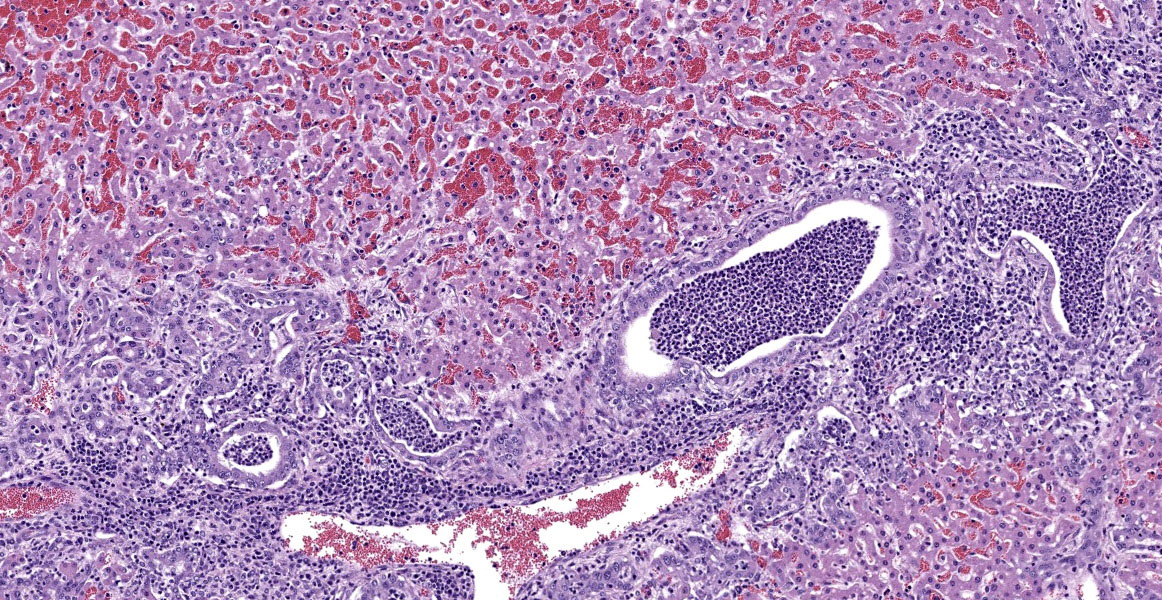
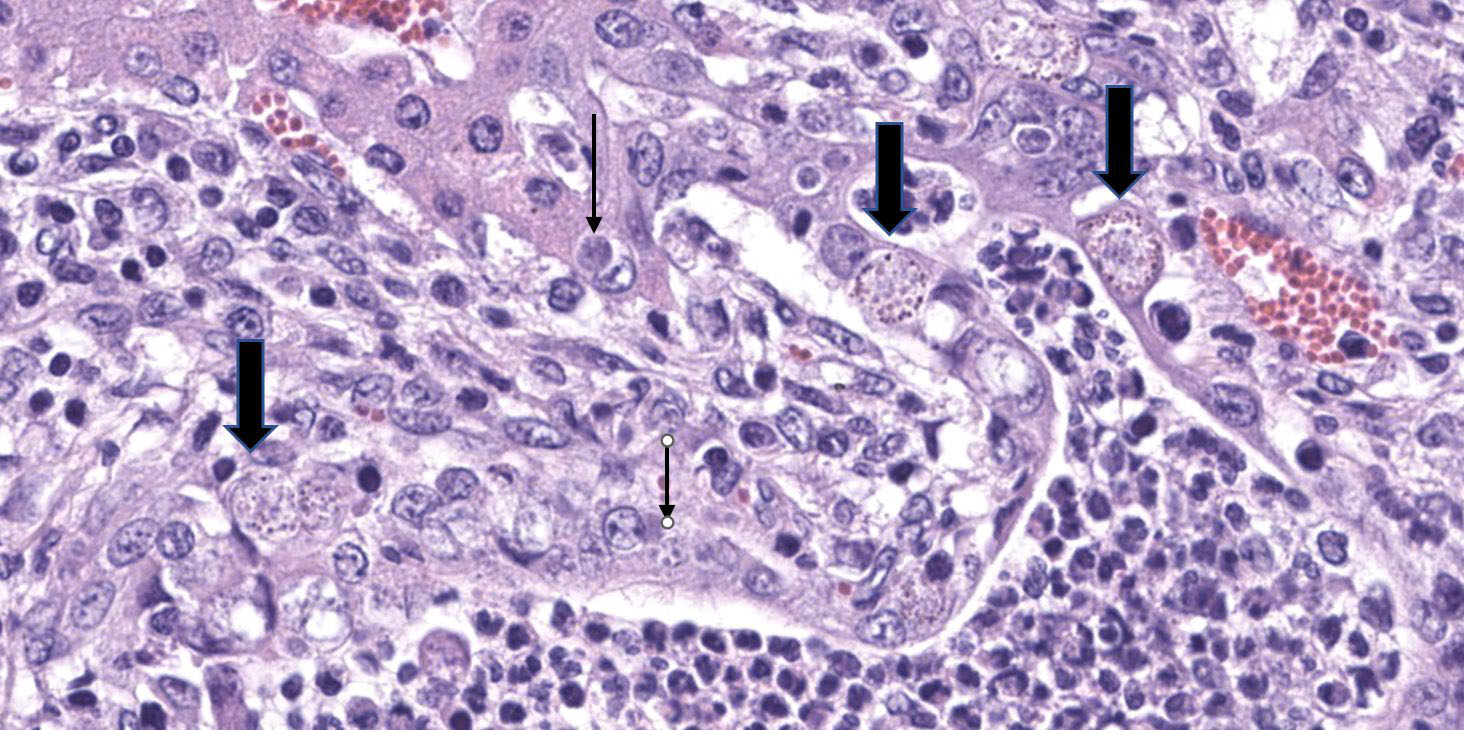
Hepatocytes are uniformly small, forming thin cords accentuated by moderate sinusoidal congestion. The parenchyma contains multifocal to coalescing, irregular, well-demarcated, variably sized, randomly distributed foci of hepatocellular degeneration and necrosis infiltrated by moderate to large numbers of predominantly neutrophils. There is widespread expansion of periportal areas by mixtures of edema, fibroplasia, proliferating bile ducts, and mixed inflammatory infiltrates predominated by lymphocytes and plasma cells, with smaller numbers of neutrophils. Biliary hyperplasia frequently links adjacent portal areas. Primarily larger bile ducts are ectatic, with lumens distended by large numbers of neutrophils, necrotic cellular debris and occasional unsporulated coccidial oocysts. The epithelium of affected bile ducts is variably attenuated, to degenerate or necrotic. Many cells have vacuolated cytoplasm that contains small coccidial meronts with merozoites, macrogametes, microgametes and developing oocysts. The lumens of some bile ducts and sinusoids contain microcolonies of small bacterial rods.
Contributor's Morphologic Diagnoses:
Liver: Necrosuppurative hepatitis, acute, multifocal to coalescing, severe, with severe suppurative cholangitis, lymphoplasmacytic pericholangitis, marked biliary hyperplasia and multiple coccidial stages
Contributor's Comment:
Gross and microscopic findings are consistent with listeriosis, a global disease of humans and other animals caused by the opportunistic, Gram-positive, intracellular bacterium, Listeria monocytogenes. Infection was confirmed by positive fluorescent antibody staining of the brain and liver. The environmentally resistant bacterium is widely distributed in soil, vegetation and in animals.1 Large numbers are present in ruminant feces and it is frequently isolated from normal tissues.2 In humans, transmission usually occurs through the consumption of contaminated food, rarely from infected animals to humans, between humans, and in utero. In foods, it survives processing technologies relying on acidic or salty conditions and can multiply at low temperatures.1 Encephalitis in ruminants is often associated with heavy silage feeding.2
Cell mediated immune responses are related to intracellular replication and have been recently reviewed, as have strategies used by the bacterium to exploit host molecular mechanisms, including translocation from cell to cell. 3,4,5 In mammalian hosts, three disease syndromes occur in relation to the bacteria's ability to cross intestinal, feto-placental and blood brain barriers. It can also survive in bile and induce biliary tract infection.6 Infection of the pregnant uterus results in abortion and frequently lethal neonatal disease. Encephalitis usually occurs in adults and is typified by microabscess formation in the brainstem. Ascending infection of the trigeminal nerve follows trauma to the oral mucosa. Septicemia, with coagulative necrosis and abscess formation occurs mainly in the livers of neonates and young juveniles following disruption of the intestinal mucosal barrier and translocation of bacteria to the submucosa and vasculature. Focal infections can include the conjunctiva, skin, mammary glands, heart, arteries, spleen, lymph nodes, joints, bone, and fascia.1,2
In addition to its biliary presence, severe intestinal coccidiosis, a common cause of diarrhea in confined young goats, may have compromised the intestinal mucosa, providing a portal of entry for L. monocytogenes into the portal circulation. Similar changes, including hepatic necrosis, biliary hyperplasia, periportal fibrosis and lymphocytic inflammation have been reported in association with hepatic coccidiosis, caused by an Eimeria sp., in a goat.7
Contributing Institution:
www.vet.uga.edu/VPP
JPC Diagnosis:
Liver: Cholangiohepatitis, suppurative and lymphoplasmacytic, chronic, diffuse, marked, with biliary hyperplasia and numerous apicomplexan meronts, gamonts, and oocysts.
JPC Comment:
Listeria monocytogenes was first described 101 years ago in a human patient and in animal species a few years later. There are 13 serovars, with three serovars (1/2a, 1/2b, 4b) being the most commonly isolated in clinical disease. As the contributor mentions, the bacterium is ubiquitous in the environment, but pathogenic serotypes have frequently been isolated from wild animals, suggesting a possible wild reservoir.2
At physiologic temperatures, L. monocytogenes actively expresses positive regulatory factor A (PrfA), allowing it to switch from an environmental to an infective lifestyle. A number of virulence factors are then expressed, including internalin A and B (InlA and InlB). These bind to non-phagocytic cell membrane receptors such as E-cadherin and induce receptor-mediated endocytosis. Alternatively, L. monocytogenes can be directly phagocytosed by phagocytic cells. Within the cytoplasmic vacuole, listeriolysin O, phospholipase A, and phospholipase B create membrane pores which allow the bacteria to escape in the cytoplasm and begin replicating. Recent evidence has also shown that the bacteria can actually remain and replicate slowly within the vacuoles. Once in the cytosol, the actin assembly-inducing protein causes the cytoskeleton to rearrange, forming actin comet-tails which propel the bacteria around the cytoplasm or into an adjacent cell.2
While ocular, cutaneous, and many rhombencephalic infections are initiated via traumatic inoculation, the method with which L. monocytogenes establishes enteric infection in ruminants is still somewhat a mystery. In mice, InlA, InlB, and Listeria adhesion protein (LAP) enable the bacteria to translocate the mucosa with minimal host reaction. Ruminants are frequently asymptomatically infected, but in clinically affected animals bacterial infection of myocytes leads to neutrophilic inflammation focused on the muscularis mucosa. In subsequent bacteremia, L. monocytogenes spreads to the spleen and liver, causing random hepatocellular necrosis, pyogranulomas, and periportal inflammation. Bacteremic spread can also spread to the placenta, causing fetoplacental infection, or to the udder, causing mastitis (though mastitis may also occur through direct inoculation). 2 The moderator and conference participants discussed the possibility that this case originated as a fetoplacental infection in which the fetus survived.
In monogastrics, blood-borne bacteria (either extracellular or in leukocytes) can cross the blood-brain barrier, causing meningitis or meningoencephalitis. In ruminants, the bacteria follows a different route of infection, crossing oral mucosa or skin, traveling up the axons of the cranial nerves, and establishing infection in the brainstem, causing rhombencephalitis.2 For additional information on the neurologic manifestation of L. monocytogenes in ruminants, we recommend reviewing WSC 21-22, Conference 12, Case 2, which featured listerial rhombencephalitis in a lamb.
The moderator and conference participants considered the distribution of hepatocellular necrosis in this case and agreed the majority of necrosis is affecting the periportal region, thus included under the umbrella of suppurative cholangiohepatitis.
References:
- Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clinical Microbiology and Infection. 2009; 16:16-23.
- Bagatella S, Tavares-Gomes L, Oevermann A. Listeria monocytogenes at the interface between ruminants and humans: A comparative pathology and pathogenesis review. Vet Pathol. 2022; 59(2):186-210.
- Charlier C, Fevre C, Travier L, et al. Listeria monocytogenes-associated biliary tract infections. Medicine. 2014; 93:1-11.
- Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. PNAS. 2011; 108:19484-19491.
- Cossart P, Lebreton A. A trip in the 'new microbiology' with the bacterial pathogen Listeria monocytogenes. FEBS Letters. 2014; 588: 2437-2445.
- Maxie MG, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. 5th Philadelphia, PA: Elsevier Saunders; 2007: 281-457.
- Schafer KA, Stevenson W, Kazacoa KR. Hepatic coccidiosis associated with hepatic necrosis in a goat. Vet Pathol. 1995; 32:723-727.
- Stavru F, Archambaud C, Cossart P. Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunological Reviews. 2011; 240:160-184.