Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 20
25 March, 2020
CASE II: 19-2535 DVD (JPC 4135747).
Signalment: One-year old female sheep
History: Two yearling sheep in a group of fifteen died within the span of two weeks. A field necropsy with limited tissue collection was performed by the primary veterinarian.
Gross Pathology: Clinician reported adequate internal fat, heavy and wet lungs, and several < 1 cm in diameter abscesses in the liver.
Laboratory results: Quantitative PCR for ovine herpesvirus 2 revealed high copy numbers of viral DNA in liver and lung (73,400 and 195,000 copies/5 ng total tissue DNA)
In situ hybridization: leukocytes demonstrate positive intranuclear staining for ovine herpesvirus 2.
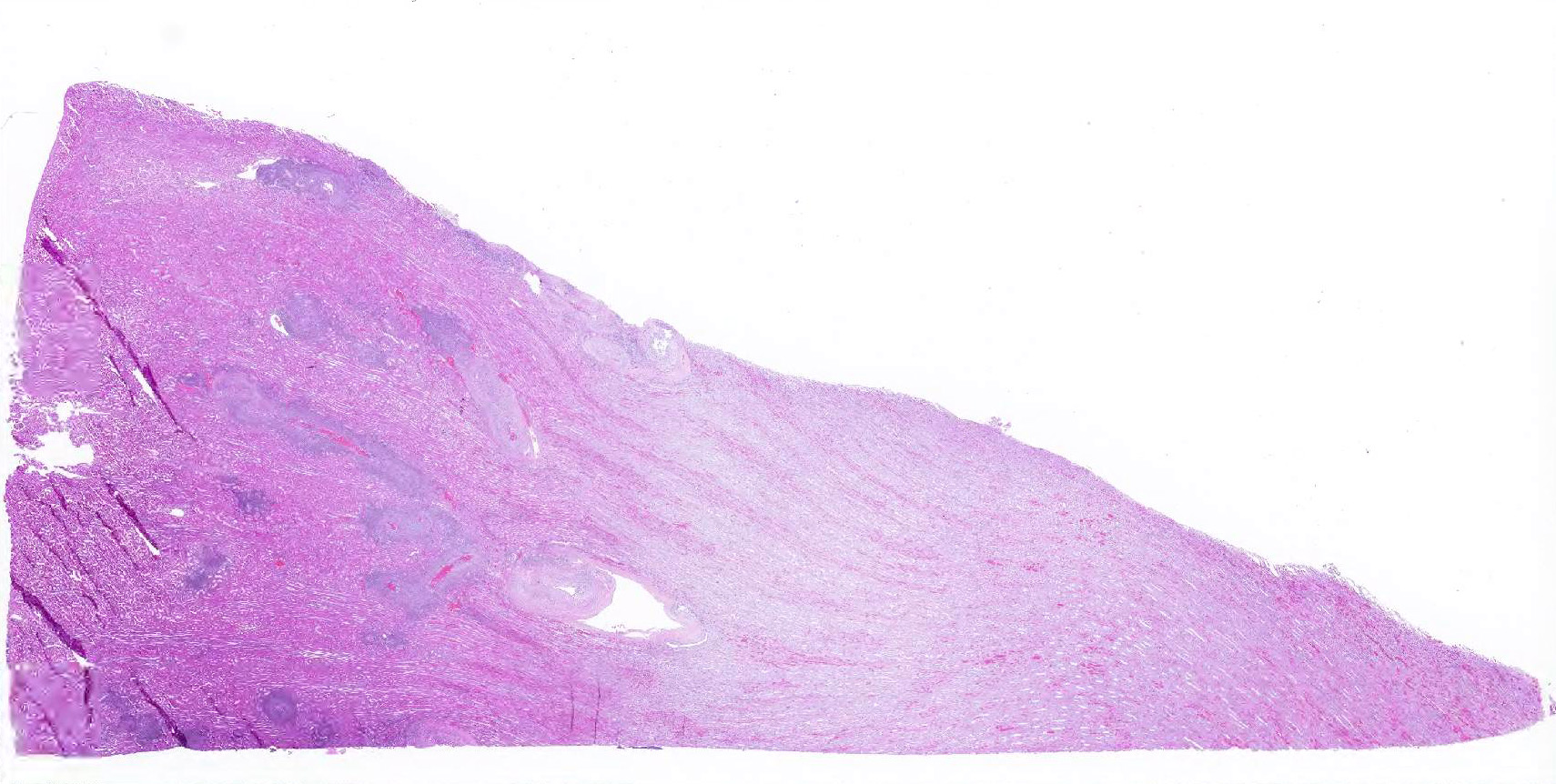
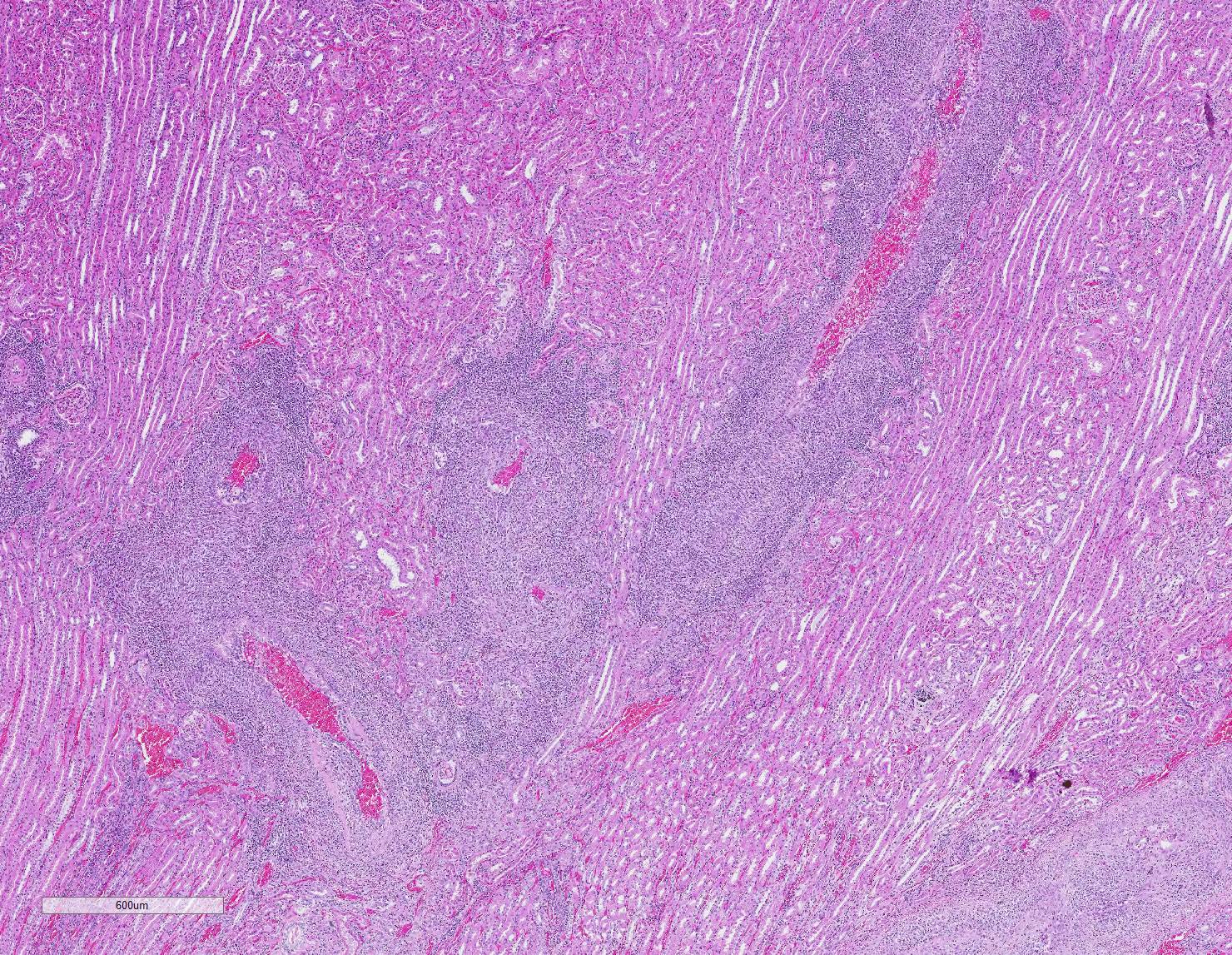
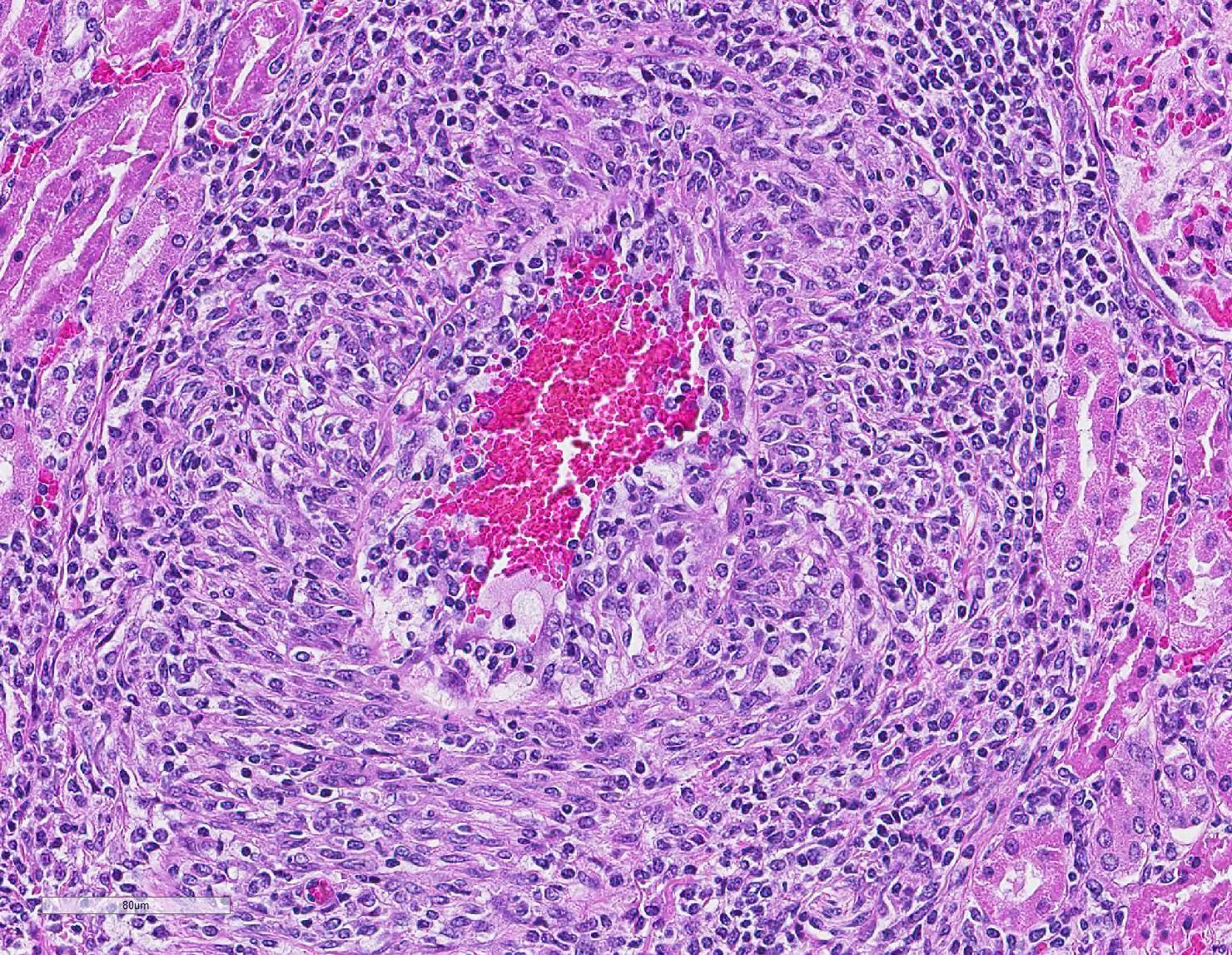
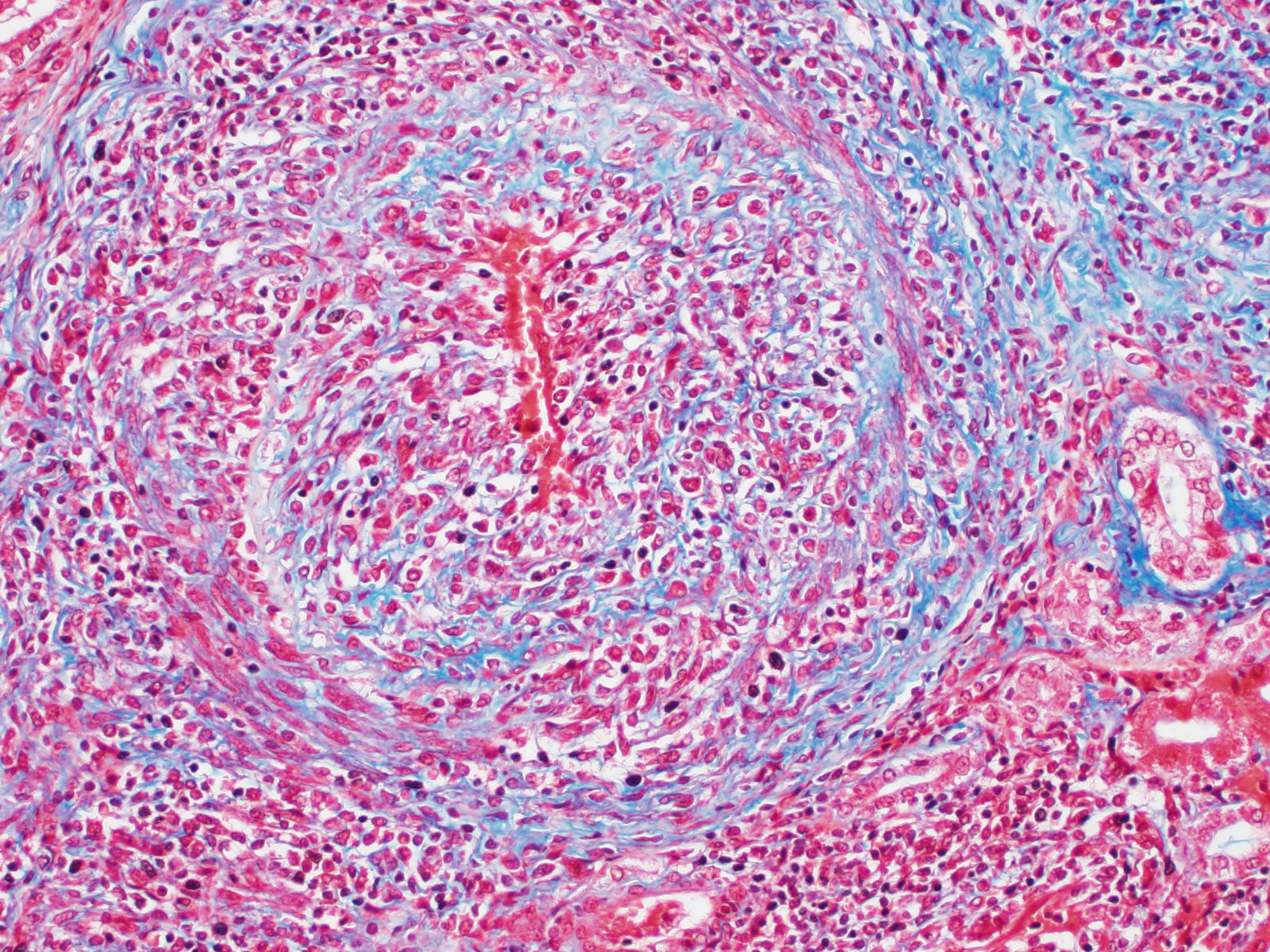
Microscopic Description: Kidney: Multifocal vessels within the renal cortex and the arcuate arteries are transmurally disrupted by moderate numbers of densely packed macrophages, lymphocytes, plasma cells, and a few neutrophils. The endothelial cells are circumferentially plump, and the internal elastic lamina of the tunica intima is multifocally interrupted or obscured by the mixed inflammation admixed with small amounts of hypereosinophilic fibrillar material, shrunken and hypereosinophilic cellular silhouettes that have pyknotic nuclei (necrosis), and eosinophilic granular debris. The tunica media and adventitia are expanded by increased numbers of plump fusiform cells and collagenous connective tissue (hypertrophy). The mixed inflammation fills the periportal connective tissue and multifocally obscures the venous silhouettes.The renal parenchyma adjacent to the affected arteries is also expanded by small to moderate numbers of lymphocytes and plasma cells.
Other tissues:
Liver: Multifocally within the portal triads or around hepatic arteries, the walls of the arteries are similarly disrupted. The mixed inflammation fills the periportal connective tissue and multifocally obscures the venous silhouettes.
Lung: A group of arteries adjacent to the cartilage of a bronchus is similarly disrupted by mixed inflammation that surrounds and extends into the wall.
Heart: A large caliber artery is disrupted by smaller amounts of mixed inflammation. All arteries have moderate to marked medial hypertrophy and thickening.
Contributor Morphologic Diagnosis:
Vasculitis, histiocytic and lymphoplasmacytic, multifocal, chronic, marked with vascular hypertrophy; liver, kidney, heart, and spleen
Contributor Comment: The presented case is consistent with systemic necrotizing vasculitis of sheep, a sporadic disease affecting individuals or clusters of sheep ranging from 5 months to 3 years old in the literature.2,7,9,10 Clinical signs of this disease may vary, and subcutaneous and joint swelling, diarrhea, chronic weight loss, and hemorrhage into body cavities with a case of aneurysmal dilation and rupture of the gastroduodenal artery have been described. Histologically, the findings are characterized by a primarily circumferential lymphocytic inflammation disrupting small- to medium-sized arteries. The inflammation and local necrosis is often most densely affecting the adventitia and outer muscular wall, but can extend to be transmural in affected vessels. Comparisons between systemic necrotizing vasculitis of sheep and polyarteritis nodosa have been described,2 and the etiology in individual cases are often speculated to be viral or immune-mediated.9,10
Recently, infection with the gammaherpesvirus ovine herpesvirus 2 (OvHV-2) has been demonstrated to be associated with systemic necrotizing vasculitis in sheep.7 Our presented case recapitulates this association by identifying OvHV-2 nucleic acid within the vascular lesion via in situ hybridization. OvHV-2, a causative agent for malignant catarrhal fever (MCF) affecting several ruminant species such as cattle, deer, and confined bison, is generally thought to be a subclinical infection in domestic sheep. Like other herpesviruses, OvHV-2 establishes persistent infection in sheep, which are the natural hosts, and the virus is shed throughout the lifetime of the host. Although complete details of the epidemiology and viral life cycle of OVH-2 infection in domestic sheep remain to be determined, the majority of lambs acquire OvHV-2 at about 10 weeks of age, and the highest levels of viral DNA is detected in nasal secretions when individuals are between 6 and 9 months of age.5 Viral DNA is detected in peripheral blood leukocytes of lambs earlier than in nasal secretions, which suggest that initial infection may occur in lymphocytes.4 Diagnosis of OVH-2 associated MCF-like syndrome in sheep is a diagnostic challenge given that the virus is readily detectable in clinically healthy animals. Supportive evidence for diagnosis in this case included characteristic vasculitis in multiple organs, quantitative PCR demonstrating high copy numbers of OVH-2 DNA in tissue, and in situ hybridization revealing OHV-2 genomic material within lesion leukocytes.
Differentials for viral-associated vasculitis in sheep are summarized in Table 1.
|
Name |
Virus |
Associated Disease |
Ancillary testing |
|
Bluetongue |
Orbivirus |
Vascular thrombosis, tissue infarction, and hemorrhage +/- vascular inflammation |
PCR of affected tissues |
|
Maedi-Visna / Ovine Progressive Pneumonia |
Small Ruminant Lentivirus |
Chronic lymphocytic pneumonitis, encephalitis, arthritis, mastitis and vasculitis |
Positive lentivirus ELISA with concurrent and characteristic lesions, IHC |
|
Border Disease |
Pestivirus |
Disseminated nodular periarteritis with a predilection for CNS tissues |
Viral isolation and/or rt-PCR |
Table 1. Differential diagnoses for viral vasculitides in sheep.1,3,6,11
Contributing Institution: Washington State University Department of Veterinary Microbiology and Pathology (https://vmp.vetmed.wsu.edu/)
Washington State Animal Disease Diagnostic Laboratory (https://waddl.vetmed.wsu.edu/)
JPC Diagnosis: Kidney, arteries: Arteritis, necrotizing and lymphohistiocytic, multifocal, chronic, severe, with moderate perivascular lymphohistiocytic nephritis.
JPC Comment: The contributor has provided a concise but thorough review of malignant catarrhal fever (MCF) in sheep. MCF has been a frequent contribution to the WSC over the years (Table 2), even before ovine herpesvirus-2 (OvH-2) was identified as a potential cause. In addition to ovine herpesvirus-2, five other MCF-inducing viruses have been identified in ungulates, including alcelaphine herpesviruses 1 and 2, caprine herpesviruses 2 and 3, and ibex malignant catarrhal virus.8
The contributor has mentioned the difficult in establishing the diagnosis of MCF in sheep. Clinically healthy sheep are thought to be widely infected with OvH-2, and would normally be PCR-positive for the present of this virus. A recent paper7 described a diagnostic protocol utilizing a combination of in-situ hybridization and quantitative PCR to compare viral nucleic acid between tissues of vasculitis and those of clinical healthy normal carriers, suggesting that the presence of ISH positivity in vascular lesions and high levels of OvH-2 DNA may be used to confirm cases of ovine MCF.7
Other unique features of MCF in the sheep have impacted the elucidation of its pathogenesis. Unlike other MCF viruses, such as alcelaphine herpesvirus-1, the virus cannot be grown in cell culture, relegating researchers to used pooled secretions from multiple lambs. In AHV-1 infection in susceptible ruminant, lymphoid hyperplasia in which the nodes are populated by large lymphocytes heralds early infection, but these changes are not seen in OVH-2 infection, in which viral distribution is limited, and lesion development is proportional to viral infection and distribution.8 One particular group has recently published a report of their development of a specific OvH-2 ISH probe with high specificity (it does not react with AHV-1, ibex-McFV, and CpHV-2). The particular probe was shown to be effective in both experimental and natural infections, even in cases with low viral copy number, and that ISH signals correlate positively with lesion severity.8
|
2018 |
1-2 |
Malignant catarrhal fever |
20180822 |
Eye |
Ox |
|
2017 |
1-2 |
Lymphocytic perivasculitis/Malignant catarrhal fever |
20170823 |
Eye |
Ox |
|
2014 |
12-3 |
Malignant catarrhal fever |
20141210 |
Skin |
Bovine |
|
2013 |
6-1 |
Malignant catarrhal fever |
20131023 |
Kidney |
Ruminant |
|
2012 |
11-3 |
Malignant catarrhal fever, caprine herpesvirus-2 |
20130109 |
Brain |
Ruminant |
|
2011 |
4-1 |
Malignant catarrhal fever |
20120125 |
Heart |
Wildlife |
|
2008 |
8-1 |
Malignant catarrhal fever |
20081105 |
artery |
Bovine |
|
2003 |
10-1 |
Malignant catarrhal fever |
12/10/2003 |
Other |
|
|
1979 |
9-2 |
Malignant catarrhal fever |
11/14/1979 |
EYE |
Bovine |
|
2002 |
3-3 |
Malignant catarrhal fever |
9/25/2002 |
Caprine |
|
|
1989 |
30-4 |
Malignant catarrhal fever |
5/23/1990 |
EYE |
Bovine |
|
1977 |
3-4 |
Malignant catarrhal fever |
10/5/1977 |
KIDNEY |
Bovine |
|
1983 |
1-3 |
Malignant catarrhal fever |
9/14/1983 |
HEART |
Bovine |
|
1983 |
1-3 |
Malignant catarrhal fever |
9/14/1983 |
KIDNEY |
Bovine |
|
1982 |
3-1 |
Malignant catarrhal fever |
9/29/1982 |
KIDNEY |
Ovine |
|
1970 |
16-1 |
Malignant catarrhal fever |
2/24/1971 |
SKIN |
Bovine |
|
1978 |
12-1 |
Malignant catarrhal fever |
1/10/1979 |
KIDNEY |
Caprine |
|
1979 |
9-2 |
Malignant catarrhal fever |
11/14/1979 |
BRAIN |
Bovine |
|
1996 |
28-1 |
Malignant catarrhal fever |
5/14/1997 |
COLON |
Bovine |
|
1989 |
30-3 |
Malignant catarrhal fever |
5/23/1990 |
KIDNEY |
Bovine |
|
2000 |
22-4 |
Malignant catarrhal fever (ovine herpesvirus) |
3/14/2001 |
Porcine |
|
|
1978 |
12-1 |
Malignant catarrhal fever |
1/10/1979 |
URINARY BLADDER |
Caprine |
|
1976 |
12-3 |
Malignant catarrhal fever |
12/15/1976 |
HEART |
Zoo/Wild Ungulate |
|
1978 |
22-3 |
Malignant catarrhal fever |
3/21/1979 |
BRAIN |
Zoo/Wild Ungulate |
|
1978 |
22-3 |
Malignant catarrhal fever |
3/21/1979 |
INTESTINE |
Zoo/Wild Ungulate |
|
1994 |
5-3 |
Malignant catarrhal fever |
10/12/1994 |
TESTICLE |
Zoo/Wild Ungulate |
|
1992 |
7-2 |
Malignant catarrhal fever |
10/28/1992 |
ESOPHAGUS |
Zoo/Wild Ungulate |
|
1991 |
30-4 |
Malignant catarrhal fever |
5/20/1992 |
KIDNEY |
Bovine |
|
1989 |
30-3 |
Malignant catarrhal fever |
5/23/1990 |
ORAL MUCOSA |
Bovine |
Table 2. WSC submissions of malignant catarrhal fever (1979-2018)
References:
- Cutlip RC, Lehmkuhl HD, Schmerr MJF, Brogden KA. Ovine progressive pneumonia (maedi-visna) in sheep. Vet Micro 1988; 17(3), 237?250.
- Ferreras MC, Benavides J, Fuertes M., García-Pariente C, Muñoz M. Pathological features of systemic necrotizing vasculitis (polyarteritis nodosa) in sheep. J Comp Path, 2013; 149(1), 74?81.
- García-Pérez A, Minguijón E, Barandika JF, Aduriz G, Povedano. Detection of Border Disease Virus in fetuses, stillbirths, and newborn lambs from natural and experimental infections. J Vet Diagn Investig, 2009; 21(3), 331?337.
- Li H, Hua, Y, Snowder G., Crawford TB. Levels of ovine herpesvirus-2 DNA in nasal secretions and blood of sheep: implications for transmission. Vet Microbiol 2001, 79(4): 301?310.
- Li, H, Taus NS, Lewis GS, Kim O, Traul DL. Shedding of ovine herpesvirus-2 in sheep nasal secretions: the predominant mode for transmission. J Clin Microbiol 2004; 42(12): 5558?5564.
- Maclachlan NJ, Drew CP, Darpel KE, & Worwa G. The pathology and pathogenesis of bluetongue. J of Comp Path 2009; 141(1): 1?16.
- Pesavento PA, Dange RB, Carmen Ferreras M, Dasjerdi A, Pérez V.. Systemic necrotizing vasculitis in sheep is associated with ovine herpesvirus 2. Vet Pathol 2019; 56(1): 87?92.
- Pesavento PA, Cunha CW, Li H, Jackson K, O?Toole, D. In situ hybridization for localization of ovine herpesvirus2, the agent of sheep-associated malignant catarrhal fever in formalin fixed tissues. Vet Pathol 2019; 56(1) 78-86.
- Rae CA. Lymphocytic enteritis and systemic vasculitis in sheep. Can Vet J 1994; 35(10): 622?625.
- Wessels M, Strugnell B, Woodger N, Peat M, La Rocca, SA. Systemic necrotizing polyarteritis in three weaned lambs from one flock. J of Vet Diagn Investig 2017; 29(5): 733?737.
- Zakarian B, Barlow RM, Rennie JC, Head, KW. Periarteritis in experimental border disease of sheep. J of Comp Path 1976; 86(3), 477?487.