Signalment:
Gross Description:
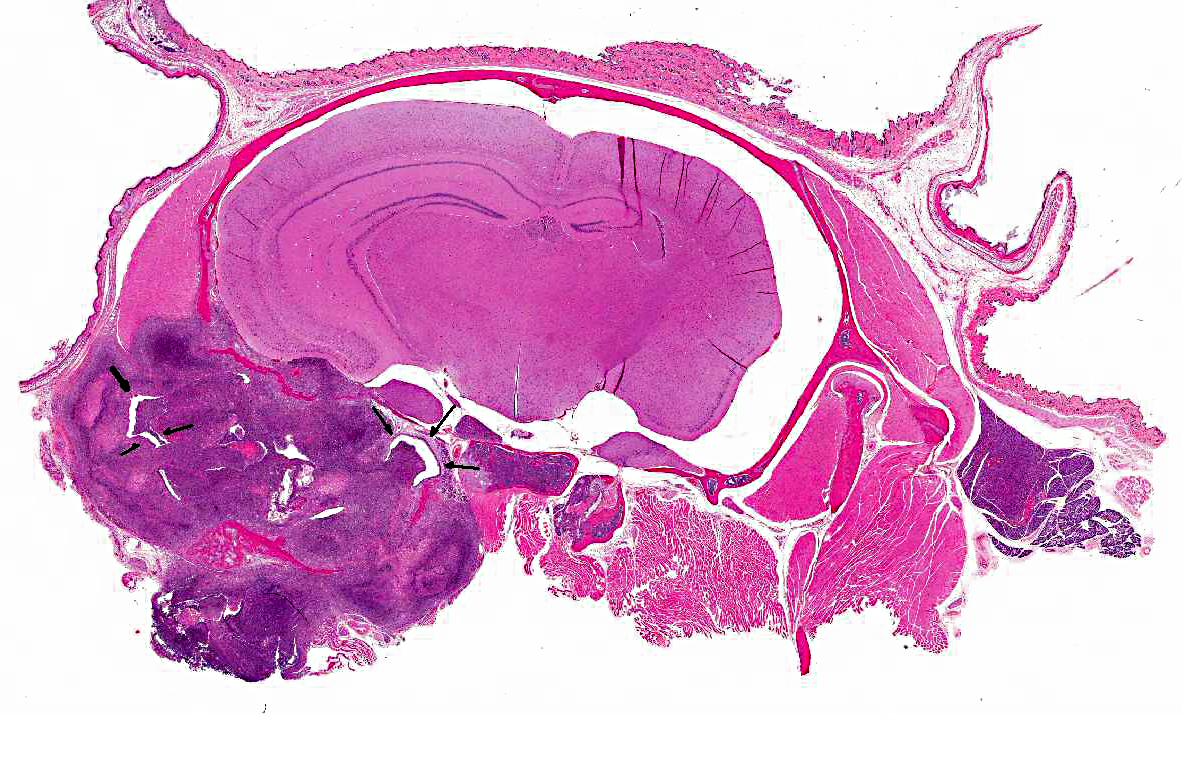
Histopathologic Description:
Morphologic Diagnosis:
1. Middle ear: Severe, chronic, suppurative, unilateral otitis media with extensive bulla osteolysis and intralesional gram negative rods.
2. Inner ear (depending on slide): Severe, chronic, suppurative, unilateral otitis interna.
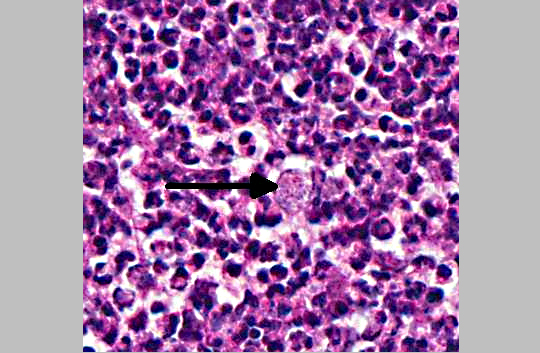
3. Brain and meninges: Severe, chronic, focal, suppurative meningoencephalitis with focal cerebral abscess and intralesional gram negative rods (secondary to otitis).
Lab Results:
Condition:
Contributor Comment:
The most common clinical presentation is head tilt, but neurologic signs such as circling and rolling have been reported in C3H mice and likely depend on the extent and severity of the lesion.(4) A history of previous chemically-induced otitis with ear damage has been found to predispose Rb/3 mice (non-susceptible strain) to audiogenic seizures.(5) In the ICR strain, otitis is manifested as mutilation of the external ear canal due to self-trauma.(6) In addition, Jeff (Jf) mutant mice are also predisposed to otitis, likely due to craniofacial abnormalities in the strain.(7)
In athymic Balb/C-derived nude mice, naturallyoccurring Sendai virus infection can cause a chronic respiratory disease characterized by rhinitis, laryngotracheitis, bronchitis/ bronchopneumonia and otitis media (usually suppurative).(8)
The pathogenesis of otitis media in the mouse is uncertain but since it is commonly found without associated otitis externa it may be a sequel of previous viral or bacterial infection of the nasopharynx with ascending infection via the Eustachian tube.(1) Acidification of the drinking water is an effective preventive measure. Tetracycline is recommended for treatment of affected animals.
Otitis media can be experimentally induced in mice by direct injection of the middle ear (often transtympanic) with human pathogenic organisms such as Streptococcus pneumonia, Haemophilus influenzae or Moraxella catharralis.9 Interleukin-8, as well as Salmonella typhimurium endotoxins can also be used to induce otitis media experimentally in mice.(10,11)
In 2004, an outbreak of otitis media associated with Burkholderia gladioli was reported in immunosuppressed mice. After an athymic nude mouse presented with head tilt and otitis, several other immunosuppressed mice in the facility presented with similar clinical and pathological findings. Culture of the middle ear of the affected mice initially yielded the phytopathogen Burkholderia cepacia, however the isolate was later identified as Burkholderia gladioli based on 16S rDNA PCR.(12)
SCID-beige mice are severely immunosuppressed because they lack mature T- and B- lymphocytes (prkdcscid mutation) and have a series of other defects affecting granulocytes, such as the lack of NK cells, reduced bacteriocidal activity of granulocytes, and decreased lysosomal enzymes in neutrophils (Beige or Bg mutation), as noted in the Jackson Laboratory Database. In light of the findings in these mice and the previously reported otitis outbreak in immunosuppressed mice caused by B.gladioli, we sent a subculture of the organism to a referral lab at the University of Missouri for PCR. B. cepacia was confirmed as the causative agent of otitis in the sick and dying Scid-Beige mice from our colony.
The genus Burkholderia was initially divided in four species: B. mallei, B. pseudomallei, B. gladioli and B.cepacia. B. mallei is the causative agent of glanders in horses, mules and donkeys; B. pseudomallei is the cause of melioidosis, a disease prevalent in Southeast Asia and Australia; B.gladioli is a primary plant pathogen but has also been isolated from the sputum of human patients with cystic fibrosis (CF); and B. cepacia causes respiratory failure in at least 20% of patients with cystic fibrosis (CF). Currently, the socalled Burkholderia cepacia-complex consists of nine genetically distinct species, all important to humans due to their ability to cause CF-related infections. B. cepacia is a small, gram negative, non-motile rod that can be transmitted from individual to individual. When patients with a mild form of cystic fibrosis are infected by B. cepacia there is a rapid decline of lung function and resulting respiratory failure with a poor outcome. Immunosuppressed CF patients that received a lung transplant are at high risk of infection with subsequent bacteremia that may result in death (cepacia syndrome).(12,14) In domestic animals, B. cepacia has only been reported as a cause of subclinical mastitis in sheep, but may have been reported in the past under the name Pseudomonas aeruginosa associated with reptile diseases and infections.(15) In the present cases, large numbers of gram-negative rods can be seen in the histologic sections submitted to conference participants. The culture of B. cepacia from blood and spleen confirms a bacteremia and explains the death of the many affected animals. Antibiotic sensitivity results indicated that the B. cepacia strain affecting these mice was resistant to some of the most common antibiotics, such as amoxicillin/clavulanic acid, ampicillin, penicillin, tetracycline, oxacillin, and erythromycin. The strain was susceptible to chloramphenicol, ciprofloxacin/ enrofloxacin, gentamicin, trimethoprim/sulfa and amikacin. The remaining colony was treated with Sulfatrim (sulfamethoxazole and trimethoprim) in the feed and recovered from the outbreak.
JPC Diagnosis:
Conference Comment:
References:
2. McGinn MD, Bean-Knudsen D, Ermel RW. Incidence of otitis media in CBA/J and CBA/CaJ mice. Hear Res. 1992;59(1):1-6.
3. Rosowski JJ, et al. The aging of the middle ear in l29S6/SvEvTac and CBA/CaJ mice: measurements of umbo velocity, hearing function, and the incidence of pathology. J Assoc Res Otolaryngol. 2003;4(3):371-83.
4. Kohn DF, MacKenzie WF. Inner ear disease characterized by rolling in C3H mice. J Am Vet Med Assoc. 1980;177(9):815-7.
5. Niaussat MM. Experimentally induced otitis and audiogenic seizure in the mouse. Experientia. 1977;33:4734.
6. Harkness JE, Wagner JE. Self-mutilation in mice associated with otitis media. Lab Anim Sci. 1975;25(3): 315-8.
7. Hardisty RE, et al. The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media. J Assoc Res Otolaryngol. 2003;130-8.
8. Ward JM, et al. Naturally-occurring Sendai virus infection of athymic nude mice. Vet Pathol. 1976;13 (1):36-46.
9. Ryan AF, et al. Mouse models of induced otitis media. Brain Res. 2006;1091(1):3-8.
10. Krekorian TD, et al. Endotoxin-induced otitis media with effusion in the mouse Immunohistochemical analysis. Acta Otolaryngol. 1990;109(3-4):288-99.
11. Johnson M, Leonard G, Kreutzer DL. Murine model of interleukin-8 induced otitis media. Laryngoscope. 1997;107(10):1405-8.
12. Foley PL, Lipuma JJ, Feldman SH. Outbreak of otitis media caused by Burkholderia gladioli infection in immunocompromised mice. Comp Med. 2004-53(1):93-9.
13. McDowell A, et al. Epidemiology of Burkholderia cepacia complex species recovered from cystic fibrosis patients: tissues related to patient segregation. J Med Microbiol. 2004;53(7):663-8.
14. Coenye T, LiPuma JJ. Molecular epidemiology of Burkholderia species. Front Biosci. 2003;8:55-67.
15. Berriatua E, et al. Outbreak of subclinical mastitis in a flock of dairy sheep associated with Burkholderia cepacia complex infection. J Clin Microbiol. 2001;39 (3):990-4.