WSC 22-23
Conference 10
Case 2:
Signalment:
5-year-9-month-old neutered female Domestic Shorthair cat, Felis catus.
History:
A 5-year-old neutered female Domestic Shorthair cat was presented to our institution following a two week history of pollakiuria and urinating in the house, which progressed to ataxia and tetraparesis. Neurologic examination localized the clinical signs to multiple spinal cord segments and an MRI of the spinal cord revealed multifocal intramedullary lesions. The cat was euthanased due to the acute onset, progressive clinical signs and was submitted for necropsy.
Gross Pathology:
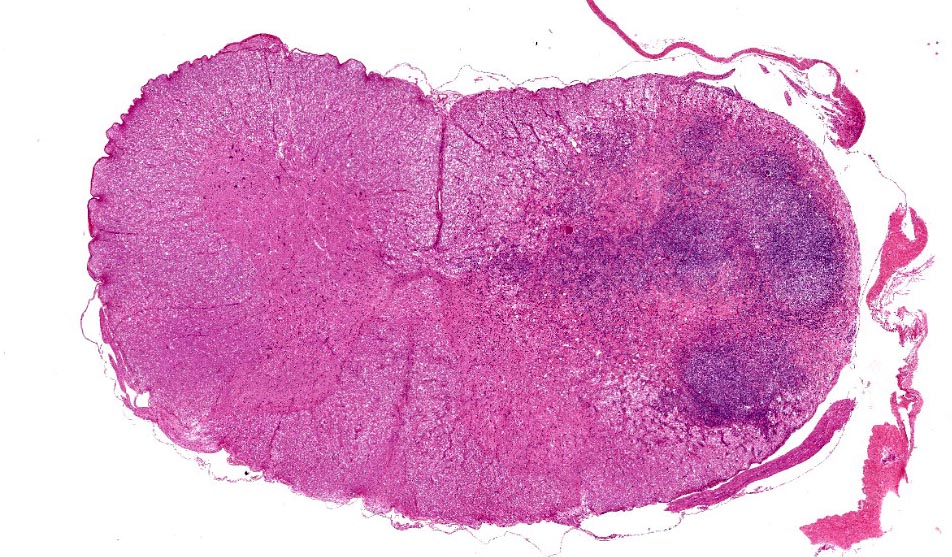
Gross examination revealed multifocal, intramedullary, grey-beige areas throughout the spinal cord. The brain was grossly unremarkable. The only other findings were those of splenic congestion and pulmonary reddening (congestion), which were attributed to barbiturate euthanasia.
Laboratory Results:
One year prior to presentation, the submitted cat was tested for FIV, FeLV and Toxoplasma spp. (testing method not provided) and was revealed to be positive for FIV and negative for FeLV and Toxoplasma spp. At the time of presentation, serology for Toxoplasma spp. revealed a high IgG titre (equal to or greater than 800, which is consistent with, though does not confirm active infection) and an IgM titre of <20.
Microscopic Description:
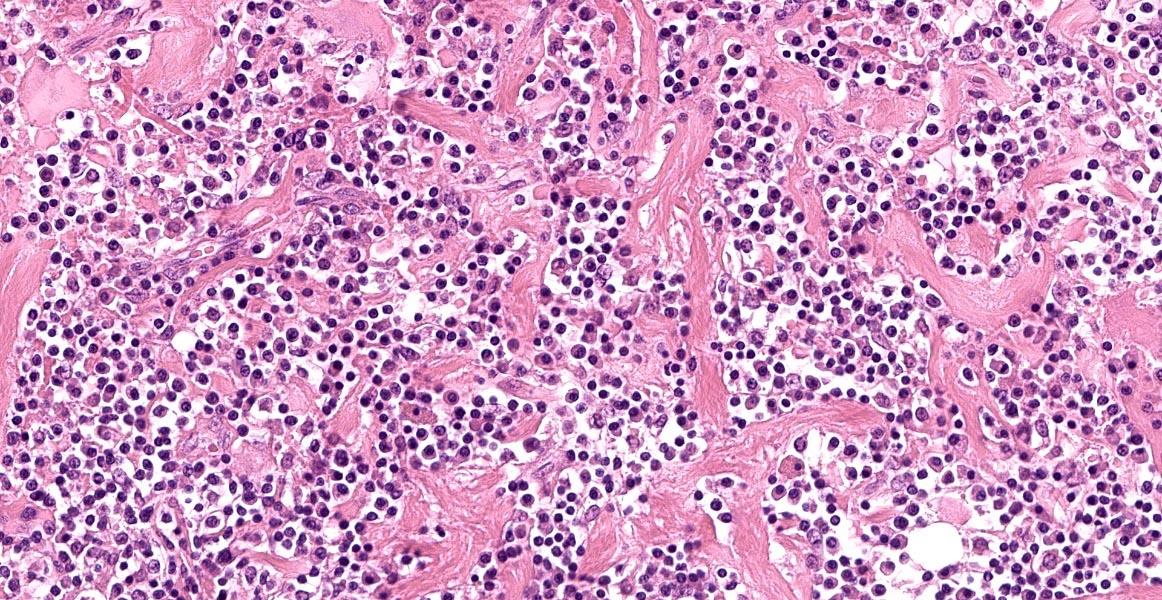
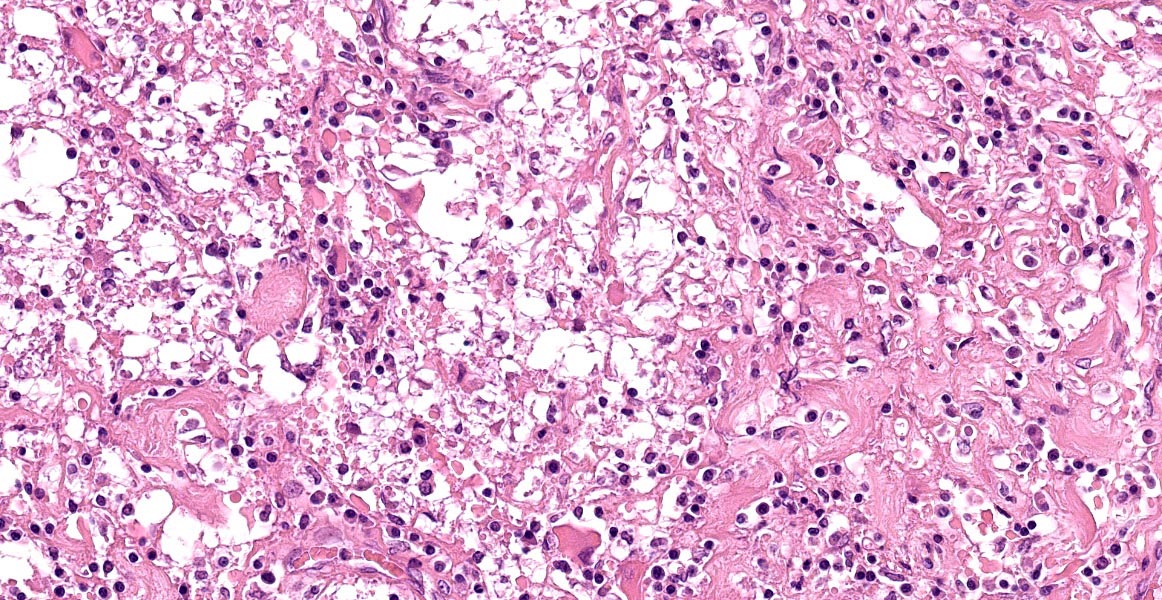
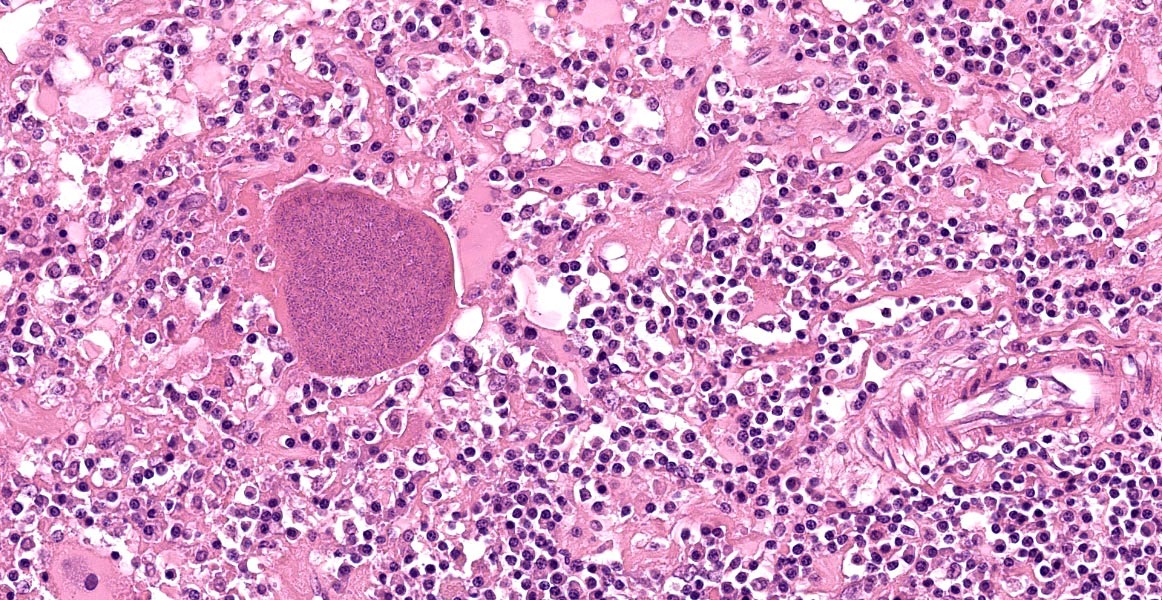
Cervical spinal cord: Unilaterally, the grey and white matter are focally extensively replaced by large numbers of infiltrating lymphocytes, plasma cells, and fewer macrophages. Neurons in this area are lost and there is amorphous eosinophilic material (necrosis). Within this area are multiple variably sized, approximately 30-60 μm diameter, eosinophilic protozoal cysts bordered by a thin (0.5 μm) capsule and containing numerous 1 μm elongate basophilic bradyzoites (presumptive Toxoplasma gondii cysts). Multifocally, there are bands of eosinophilic, fibrillar material (glial scars) and increased numbers of glial cells including gemistocytic astrocytes characterized by large, swollen, eosinophilic cytoplasm, and peripheral round nuclei and gitter cells with foamy cytoplasm and pyknotic nuclei. The adjacent white matter is rarefied and degenerate with distended axon sheaths, occasionally containing swollen, rounded and eosinophilic axons (spheroids) or associated with gitter cells (ellipsoids). Endothelial cells are lined by prominent (reactive) nuclei and Virchow-Robin spaces are expanded by lymphocytes and plasma cells (perivascular cuffing).
Contributor’s Morphologic Diagnoses:
Spinal cord, cervical; focally extensive necrotizing and lymphoplasmacytic myelitis with intra-lesional protozoal cysts (Toxoplasma gondii)
Contributor’s Comment:
The histologic features in this case are consistent with protozoal necrotizing myelitis due to Toxoplasma spp.
Toxoplasma spp. are protozoan parasites able to infect a diverse range of species including humans, nonhuman primates, birds and a large number of mammals12. Domestic and non-domestic cats are the definitive host of Toxoplasma spp. and become infected following ingestion of asexual stages in tissue or oocysts, or less frequently, congenitally12. Organisms then undergo asexual development in intestinal epithelium and can disseminate to a range of other tissues, including the lungs, liver, heart, and nervous system as free organisms or via leukocyte trafficking in lymphocytes or macrophages11. Tachyzoites are responsible for the acute presentation of toxoplasmosis and can continue to replicate indefinitely12.
Typical histologic changes in the central nervous system of cats with active toxoplasmosis include non-suppurative meningoencephalitis and necrosis. Infection in utero can cause placentitis, myocardial injury or systemic inflammation, which in turn lead to necrosis in the brainstem and cerebrocortical white matter12. Cell necrosis also occurs directly as a result of intracellular replication of tachyzoites in target cells and subsequent cell lysis or as a consequence of the host immune response. T-cell lymphocytes kill infected cells directly or via interferon-gamma-mediated activation of microglia and astrocytes11.
Chronic toxoplasmosis is characterized by a dormant stage in which bradyzoites are enclosed within cyst walls and divide slowly. Such bradyzoite-containing cysts are typically found in the brain, skeletal muscle, and myocardium in chronically infected cats12. Cyst walls are poorly immunogenic and protect bradyzoites from the host’s immune system, allowing them to remain dormant. Thus, there is usually little, if any, associated inflammation. However, in this case, areas of necrosis and inflammation in the spinal cord were associated with bradyzoite-containing cysts.
The finding of bradyzoite cysts within areas of inflammation and necrosis in feline spinal cords has been documented in several case reports of Toxoplasma or Toxoplasma-like infection.1,6 It is thought that the inflammation in these reports was the result of cyst rupture and zoite release following reactivation of latent infection. From their findings, Lyndsay et al. suggest that when toxoplasmosis in cats manifests as neurologic signs alone, without concurrent systemic signs, it is more frequently due to reactivation than acute infection.10
Reactivation of Toxoplasma has been reported in immunodeficient or immunosuppressed cats infected with Feline Immunodeficiency Virus (FIV) (as in this case) or receiving immunosuppressive therapy.2 However, the relationship between concurrent FIV infection and toxoplasmosis is not clear. A recent study by Dubey et al. did not find a correlation between naturally infected cats that were seropositive for FIV and T. gondii.8
Contributing Institution:
Pathobiology and Population Sciences
Royal Veterinary College
Hawkshead Lane, North Mymms
Hertfordshire
AL9 7TA
United Kingdom
JPC Diagnosis:
Cervical spinal cord: Myelitis, histiocytic and lymphoplasmacytic, unilateral, focally extensive, severe, with gliosis and intracellular protozoal cysts.
JPC Comment:
Toxoplasma gondii was first described in 1908 by researchers investigating Leishmania in gundis, a type of rodent native to Tunisia.5 The name was derived from the words Toxo-, which means arc and refers to the crescentic shape of zoites; -plasma, meaning life; and gondii, referring to the species it was first identified in.5 The first congenital case of T. gondii was described in 1938 in a human neonate who died after developing encephalitis and retinitis, and the first case of infection in a cat was diagnosed in 1942.4,5 In the 1950s, it was discovered to be the causative agent of abortion in ewes in New Zealand (referred to as “type II abortions”).5 Around this time, researchers also discovered links between T. gondii infection and the consumption of raw meat and exposure to cat feces, but it wasn’t until 1970 that the key to T. gondii spread was uncovered: sexual reproduction and shedding of oocysts by felids.4
Our knowledge of T. gondii has expanded drastically since its initial discovery, and the protozoa is now known to have a worldwide distribution and a wide host range.5 Felids, both domestic and wild, are the definitive hosts and become infected after ingesting meat infected with bradyzoite-laden cysts.4 Cats can also be infected by ingesting infected oocytes in feces, but this is less common.4 There are a wide range of intermediate hosts with significant inter-species variability in morbidity. Australian marsupials and New World monkeys are particularly susceptible to infection, while horses, cattle, and rats seem resistent.4,11 Other species which can be infected include other mammals, birds. fish, amphibians, and reptiles.11 Outbreaks in humans have been linked to drinking contaminated water, and it has been speculated that runoff may be the cause of recent infections documented in marine mammals.8,11
In cats, acute systemic infections most commonly result in pulmonary, nervous, hepatic, cardiac, and ocular lesions, and, as the contributor mentions, reactivated infections are more commonly restricted to the nervous or ophthalmic systems.8 Neospora caninum and Sarcocystis neurona are less common causes of encephalitis and myeloencephalitis in cats.3,7 These organisms are difficult to impossible to distinguish using light microscopy and should be considered as differential diagnoses in this case. PCR, immunohistochemistry, and serology (as conducted in this case) are generally used to differentiate between these protozoa.13
References:
- Alves L, Gorgas D, Vandevelde M, Gandini G, Henke D. Segmental meningomyelitis in 2 cats caused by Toxoplasma gondii. J Vet Intern Med. 2011;25:148-152.
- Barrs VR, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporine therapy. Aust Vet J. 2006;84:30-35.
- Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. 386-389.
- Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. I Journ Parasit. 2009; 39: 887-882.
- Dubey JP. The History of Toxoplasma gondii – The First 100 Years. J Eukaryot Microbiol. 2008; 55(6): 467-475.
- Dubey JP, Fenner, WR. Clinical segmental myelitis associated with an unidentified Toxoplasma-like parasite in a cat. J Vet Diagn Invest. 1993;5:472-480.
- Dubey JP, Greene CE. Enteric Coccidiosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO : Elsevier; 2012:837.
- Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier; 2012:806-821.
- Dubey JP, Lappin MR, Kowk OCH, et al. Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp., Feline Immunodeficiency Virus, and Feline Leukemia Virus Infections in Cats from Grenada, West Indies. J Parasitol. 2009;95(5):1129-1133.
- Lindsay SA, Barrs VR, Child G, Beatty JA, Krockenberger MB. Myelitis due to reactivated spinal toxoplasmosis in a cat. J Fel Med Surg. 2010;12:818-821.
- Miller AD, Zachary, JF. Nervous System. In: Zachary, JF ed. Pathologic Basis of Veterinary Disease. 6th ed. St Louis: Elsevier; 2017: 844-845.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie GM, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. 6th ed. St. Louis, MO: Elsevier; 2016: 117-243.
- Vadnevelde M, Higgins RJ, Oevermann A. Veterinary Neuropathology: Essentials of Theory and Practice. 1st ed. Ames, IO: John Wiley & Sons, LTD. 2012; 69.