Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 8
October 25th, 2017
CASE IV: H17-0145J (JPC 4100435).
Signalment: Unknown age, female, Vicugna pacos, alpaca.
History: A single deceased, female alpaca, which had recently given birth to a premature cria, was submitted to the anatomic pathology service. 23 alpacas had died on the property in the previous week. All ages of animals were affected. Death occurred within approximately 48h after the onset of clinical signs, which included ataxia, respiratory distress, dark brown bloody nasal and oral discharge and abortions. Three deceased alpacas were submitted a day earlier to the Department of Agriculture and Food Western Australia. The farmer reported a history of heavy rainfall 7-10 days prior to the animals becoming ill, and that the property had not had animals on it for 5 years before the alpacas were moved on to the property.
The submitted alpaca was previously treated at The Animal Hospital at Murdoch University with intranasal oxygen and intravenous fluids, however, it was found deceased the next morning and submitted to Murdoch University pathology department.
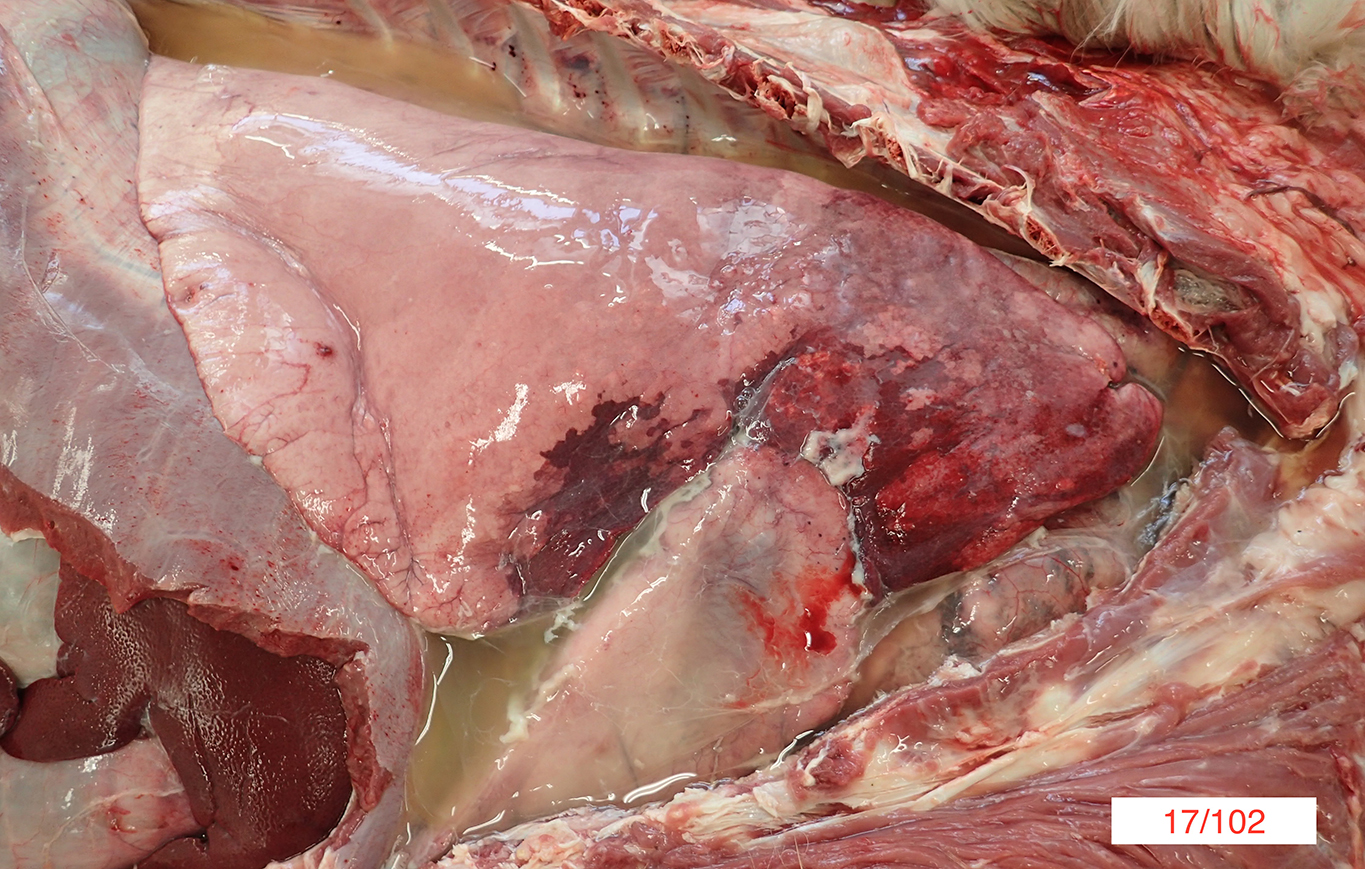
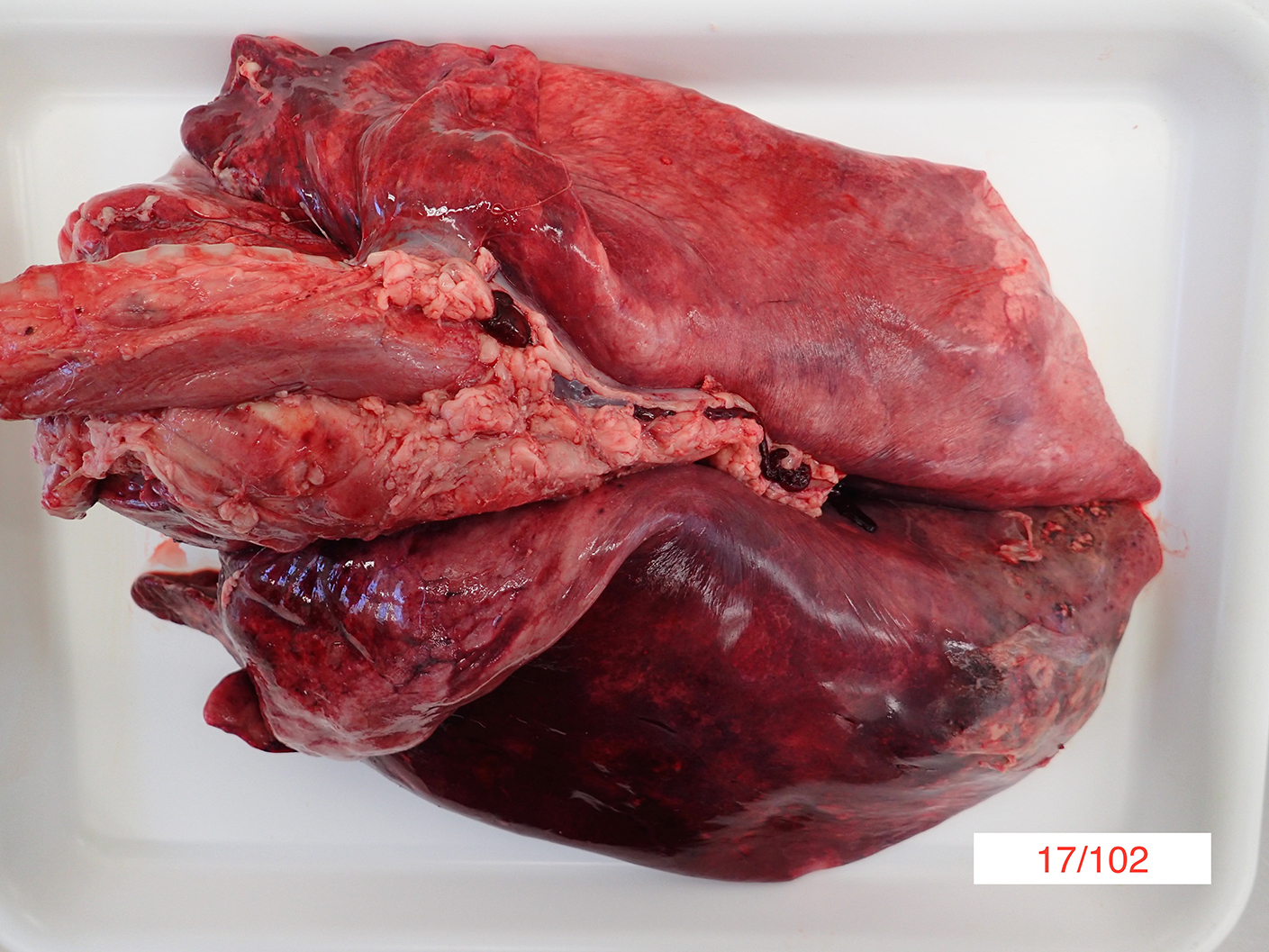
Gross Pathology: On gross necropsy examination the alpaca was in lean body condition (2/5) and had hundreds of approximately 1-3mm diameter dark red foci throughout its oral and conjunctival mucosae, subcutaneous tissues and thoracic pleura.
Diffusely the left lung lobes were mottled dark red to black, firm and oozed a moderate amount of dark red, watery, turbid fluid. Multifocally scattered throughout the left lung lobes there were fewer than ten, multifocal to coalescing, approximately 1-10cm diameter, firm, irregularly shaped nodules, which ranged from pale tan to black. Approximately 10-20% of the cranioventral region of the right lung lobes was discoloured dark red and firm and oozed a small amount of dark red watery fluid. Approximately 600mL of pale yellow, clear fluid, which contained moderate amounts of pale tan, easily broken down, stringy material was present within the thoracic cavity.
Approximately 500mL of pale yellow, clear fluid, which contained moderate amounts of pale tan, easily broken down, stringy material was present within the abdomen. Approximately 20mL of a similar fluid was present within the pericardial space.
The duodenal mesenteric lymph nodes measured approximately 3cm x 4cm x 5cm to 4cm x 4cm x 7cm respectively with replacement of the normal architecture by moderate amounts of pale tan, thick, turbid fluid and pale tan, firm to crumbly material.
Laboratory results: Burkholderia pseudomallei was isolated from aseptically collected samples of both the lung and mesenteric lymph node.
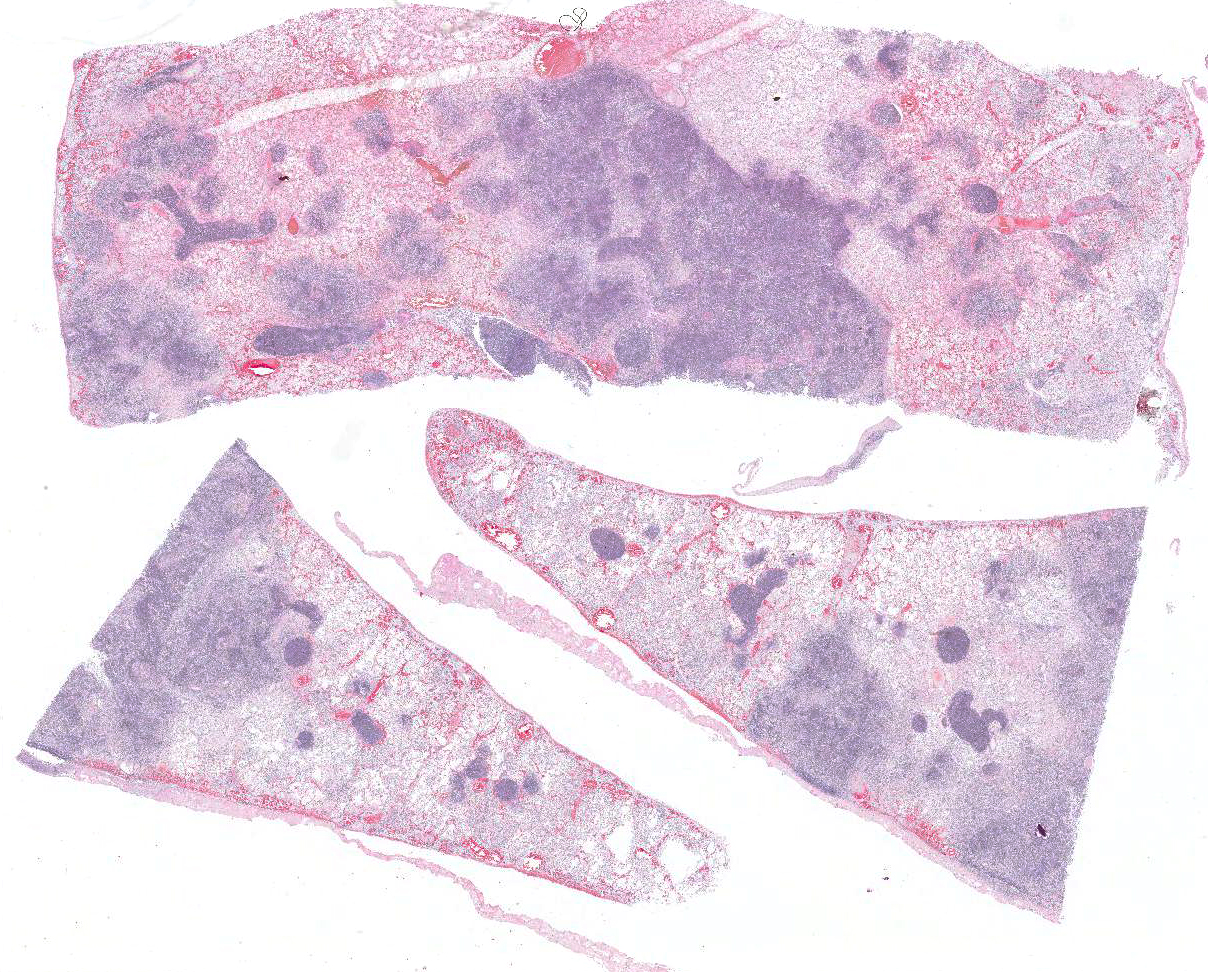
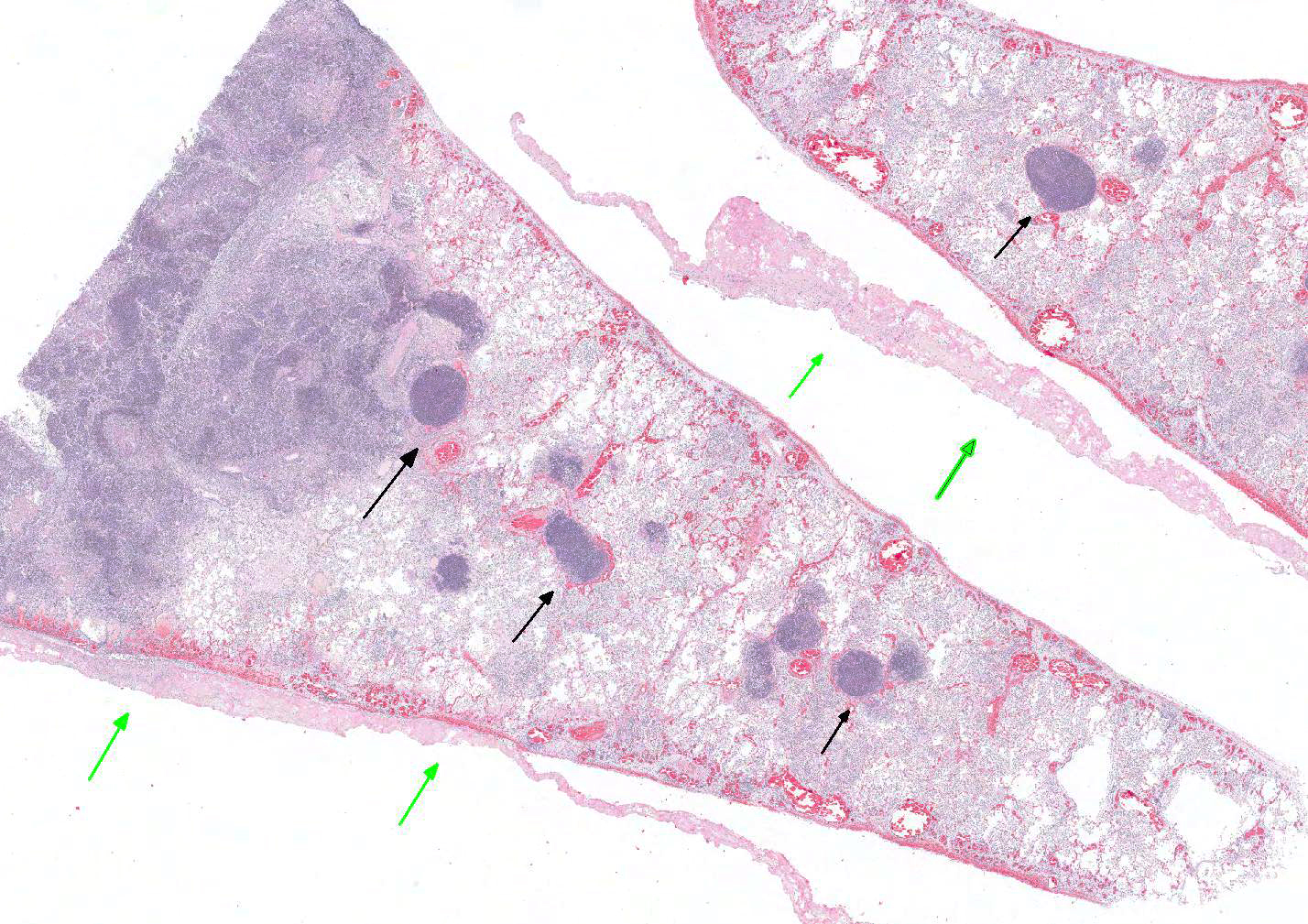
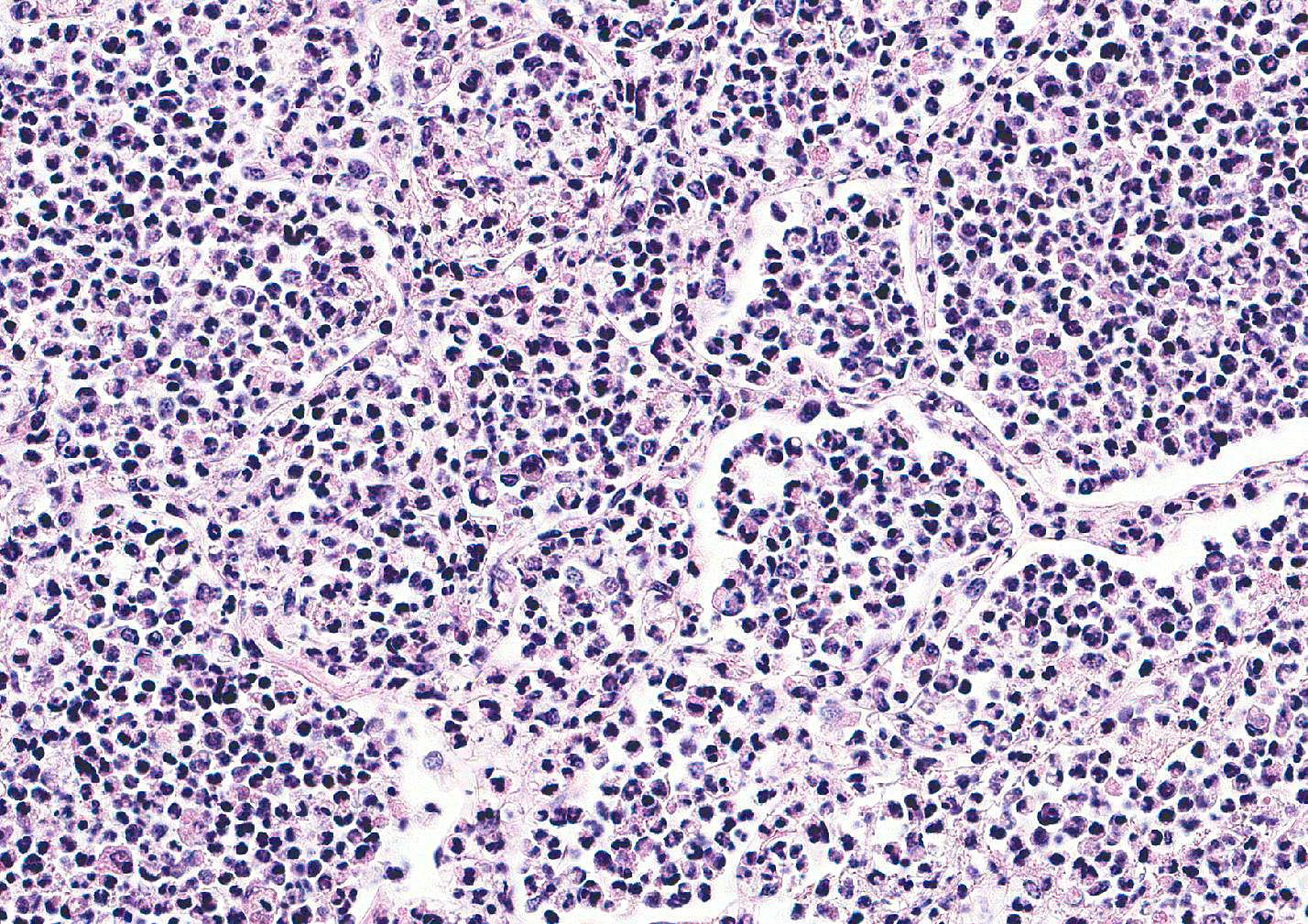
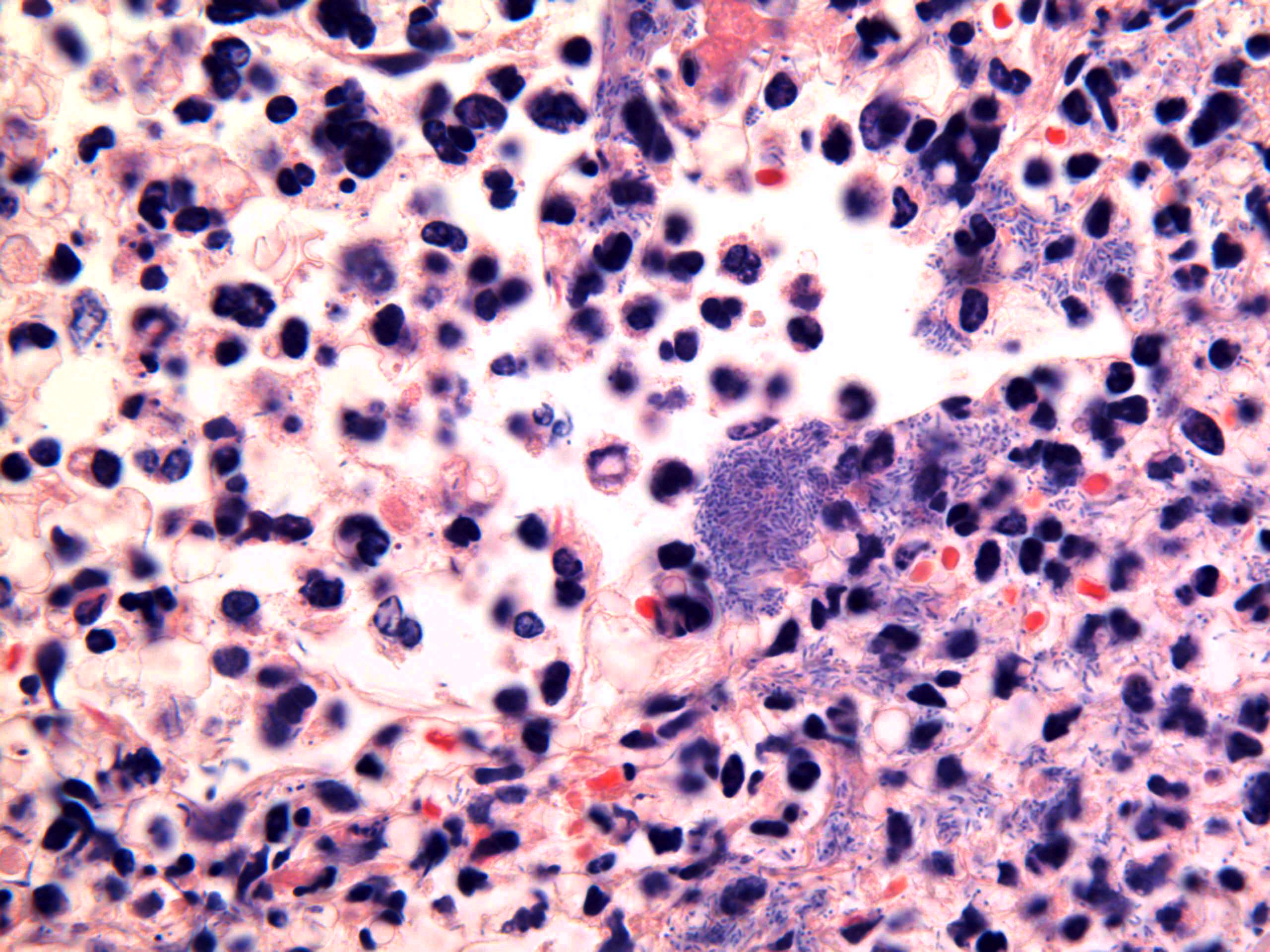
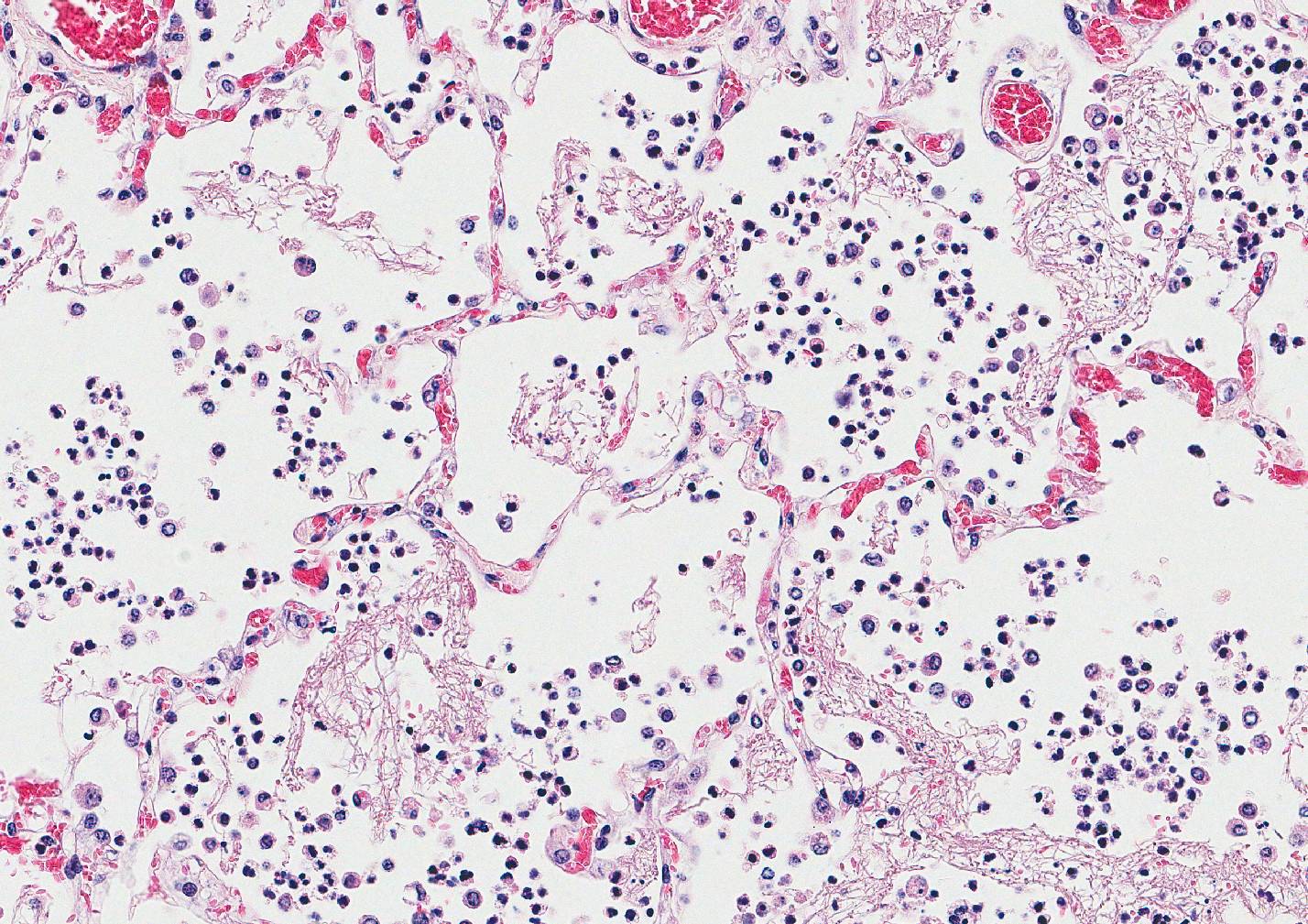
Microscopic Description: Lung: Multifocally disrupting and infiltrating the pulmonary architecture are variably dense aggregates of large numbers of frequently degenerate neutrophils, low numbers of foamy macrophages and large amounts of pale eosinophilic fibrillar material (fibrin) intermixed with moderate amounts of amorphous eosinophilic and karyorrhectic debris (necrosis). Multifocally present within the cytoplasm of occasional macrophages and occasionally free within the tissue there are low numbers of approximately 2µm in length, 1µm in diameter, gram-negative bacilli. The pulmonary pleura is multifocally expanded and infiltrated by large amounts of pale eosinophilic fibrillar material (fibrin), intermixed with moderate numbers of degenerate neutrophils and fewer lymphocytes and macrophages. Within the remaining pulmonary parenchyma,alveoli contain and are occasionally filled by moderate amounts of a similar inflammatory infiltrate intermixed with moderate numbers of extravasated erythrocytes (haemorrhage) and moderate numbers of macrophages, which frequently contain one to two intracytoplasmic erythrocytes.
Contributors Morphologic Diagnosis: Lung: Severe, acute, multifocal to coalescing, necrosuppurative and fibrinous bronchopneumonia with haemorrhage, fibrinous pleuritis and intra- and extracellular gram-negative bacilli.
Contributors Comment: This case represents a case of fatal melioidosis, caused by the bacterium Burkholderia pseudomallei, in an unusual geographic location within Australia. In addition to the alpaca submitted to our diagnostic necropsy service, three additional alpacas from the same property were also involved in the initial outbreak and confirmed to have similar gross and histopathologic changes with a positive culture of B. pseudomallei. Furthermore, a single macaw from a neighboring property, which was co-infected with Chlamydia psittaci, was also found dead with similar gross necropsy changes and positive B. pseudomallei culture.
Differentials for pneumonia reported in alpacas, particularly neonatal alpacas, include bovine respiratory syncytial virus, Parainfluenza type 3 virus, Pasteurella multocida, and Mannheimia haemolytica. The aforementioned infectious agents have been described in crias to elicit variable gross and histologic pneumonias ranging in severity (mild to severe), distribution (focal to diffuse) and pathologic processes (e.g. necrotizing, fibrinous, suppurative).14 Additionally bovine diarrhea virus, bovine herpesvirus-1, influenza virus A and Mycoplasma spp. have been shown to be associated with lower respiratory diseases in alpacas.14
Melioidosis is typically considered endemic to northern Australia and Southeast Asia with endemic and sporadic cases also reported in various countries within South and North America, Africa, the Middle East and Oceania in addition to China, India Puerto Rico, Haiti, Guadeloupe, Haiti and Martinique.6,15 The location of the outbreak reported in this case is unusual, considering its geographic occurrence within the southwestern wheatbelt region of Western Australia. Further investigation revealed historical evidence of previous outbreaks of melioidosis on the same property. It is thought that the reported heavy rainfall, atypical for the season, immediately prior to this outbreak lead to a re-emergence of the bacterium.
B. pseudomallei is a saprophytic gram-negative motile non-spore forming bacillus which is able to survive in soil and water for many years and in adverse environmental conditions including low pH and high temperatures.7,10 Infection typically occurs from inhalation of contaminated dust, ingestion of contaminated water or introduction of contaminated soil or water into skin wounds.10 Horizontal transmission between infected animals and people has not been reported; however, vertical transmission through the placenta has been reported.9 Whilst no evidence of zoonotic transmission for B. pseudomallei has been demonstrated, indirect transmission from animals and animal products has been argued to pose a possible risk to human health.8
Incidence of infections within endemic areas is highest within monsoonal seasons, following high rainfall events. 1,2 The hypothesized cause of this is the contribution of warm, wet conditions to the rapid proliferation of the bacterium within the soil after it has been brought to the surface by a rising water table. 1 Infection has been reported in humans, domestic and non-domestic animal species.13 Reported cases in domestic species are most prevalent in ruminants and swine, with swine having been reported to be less susceptible to the disease than goats and sheep.11,13 Additional reports of melioidosis within the literature include horses, cats, dogs, iguanas, rodents, camels, alpacas, horses, deer, tree kangaroos, wallabies, koalas, crocodiles, numerous avian species, captive marine mammals and a number of non-human primates.12,13,16,17 Clinical disease and necropsy findings in all species are highly variable, depending on the infection entry site, bacterial strain and immune status of the animal.15 In pigs, melioidosis is frequently asymptomatic with lesions detected upon routine abattoir inspection of carcasses.11 In other species, pneumonia and chronic, localized infections are most commonly reported, however bacteremia leading to septic shock, neurologic symptoms and mastitis have also been documented.15,16 Gross and histopathologic changes can range from acute necrosuppurative foci present within multiple organs to chronic, well-formed granulomas.16 It is important to note that B. pseudomallei can be misidentified as a contaminant, as Pseudomonas spp. or other non-pathogenic Burkholderia spp. by standard identification methods.9 Furthermore B. pseudomallei do not typically colonize the skin and, thus, identification of the organisms should always be considered as a true infection.9
Whilst any animal is potentially susceptible to infection, increased risk of infection, particularly fatal infection, with B. pseudomallei is found in people with underlying medical conditions including diabetes mellitus, renal dysfunction, immunosuppression, excessive alcohol intake, pulmonary disease, malnutrition and thalassemia.9,15 Infection in people can undergo a period of latency, which has been reported to last up to 62 years, with reactivation of latent melioidosis to clinical disease which is well documented in patients with immunosuppression.3,10 An investigation into the immunologic factors inferring susceptibility to B. pseudomallei has been performed using BALB/c-C57BL/6 mouse models. Major findings in this study demonstrated in vivo depletion of macrophages rendered C57BL/6 mice highly susceptible to intranasal infection with B. pseudomallei and that increased bacterial loads and higher mortality rates were observed for TNF-a, TNFR1 and TNFR2 knockout mice.4
Given the above information, the number of alpacas affected, the severity of disease and the involvement of a potentially immunosuppressed macaw in the outbreak has raised a question regarding the susceptibility, including the mechanisms of susceptibility, of alpacas to this disease. This would require further investigation and is a topic for potential future research.
JPC Diagnosis: Lung: Bronchopneumonia, necrosuppurative and fibrinous, diffuse, severe, with fibrinous pleuritis, alveolar necrosis, and rare intra-and extracellular bacilli, Vicugna pacos, alpaca.
Conference
Comment: Burkholderia
pseudomallei (details described above) result in either acute or chronic
disease patterns. In acute disease, which is more common in younger animals, initially
the lungs are infected followed by systemic infection. The chronic pattern is
more frequent and is characterized by abscesses in multiple organs which are
often incidental findings at slaughter. Due to the zoonotic risk, care must be
taken at slaughter, as these abscesses are not characteristic and are often
mistaken for caseous lymphadenitis (Corynebacterium
pseudotuberculosis) and glanders (Burkholderia
mallei). Brain lesions must be differentiated from listeriosis. Besides its
endotoxin (typical of a gram-negative bacterium), B. mallei has several virulence factors including: malleobactin (an
iron-scavenging protein), secreted proteases to degrade tissues, a
polysaccharide capsule that protects them from phagocytic killing, and Burkholderia lethal factor 1 which
inhibits translation and causes death of host cells.
Discussion in this case also covered the two superimposed lesions within the
submitted tissue both a necrotizing bronchopneumonia as well as a diffuse
fibrinous pleuropneumonia, both likely resulting from the presence of B. pseudomallei. Protease secretion of the bacterium results
in the severe bronchopneumonia; killing of the bacteria results in liberation
of endotoxin, diffuse damage to the pulmonary and pleural endothelium, and a
fibrinous interstitial pneumonia and pleuritis.
http://www.murdoch.edu.au/School-of-Veterinary-and-Life-Sciences/
References:
1. Baker AL, Ezzahir J, Gardiner C, Shipton W, Warner JM. Environmental attributes influencing the distribution of Burkholderia pseudomallei in Northern Australia. PloS one. 2015;10(9):e0138953.
2. Baker AL, Warner JM. Burkholderia pseudomallei is frequently detected in groundwater that discharges to major watercourses in northern Australia. Folia microbiologica. 2016;61(4):301-305.
3. Barnes JL, Ketheesan N. Development of protective immunity in a murine model of melioidosis is influenced by the source of Burkholderia pseudomallei antigens. Immunology and cell biology. 2007;85(7):551-557.
4. Barnes JL, Williams NL, Ketheesan N. Susceptibility to Burkholderia pseudomallei is associated with host immune responses involving tumor necrosis factor receptor-1 (TNFR1) and TNF receptor-2 (TNFR2). FEMS Immunology & Medical Microbiology. 2008;52(3):379-388.
5. Caswell JL, Williams KJ. Respiratory system. In : Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th ed. Vol. 2. Philadelphia, PA: Saunders Elsevier; 2016; 563.
6. Elschner MC, Hnizdo J, Stamm I, El-Adawy H, Mertens K, Melzer F. Isolation of the highly pathogenic and zoonotic agent Burkholderia pseudomallei from a pet green Iguana in Prague, Czech Republic. BMC veterinary research. 2014;10(1):283.
7. Galyov EE, Brett PJ, DeShazer D. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annual review of microbiology. 2010;64:495-517.
8. Höger A, Mayo M, Price EP, Theobald V, Harrington G, Machunter B, et al. The melioidosis agent Burkholderia pseudomallei and related opportunistic pathogens detected in faecal matter of wildlife and livestock in northern Australia. Epidemiology and infection. 2016;144(09):1924-1932.
9. Kelser EA, Melioidosis: A Greater Threat Than Previously Suspected?, Microbes and Infection (2016), doi: 10.1016/j.micinf.2016.07.001.
10. Lee S-H, Chong C-E, Lim B-S, Chai S-J, Sam K-K, Mohamed R, et al. Burkholderia pseudomallei animal and human isolates from Malaysia exhibit different phenotypic characteristics. Diagnostic microbiology and infectious disease. 2007;58(3):263-270.
11. Millan JM, Mayo M, Gal D, Janmaat A, Currie BJ. Clinical variation in melioidosis in pigs with clonal infection following possible environmental contamination from bore water. The Veterinary Journal. 2007;174(1):200-202.
12. Parkes HM, Shilton CM, Jerrett IV, Benedict S, Spratt BG, Godoy D, et al. Primary ocular melioidosis due to a single genotype of Burkholderia pseudomallei in two cats from Arnhem Land in the Northern Territory of Australia. Journal of feline medicine and surgery. 2009;11(10):856-863.
13. Ritter J, Sanchez S, Jones T, Zaki S, Drew C. Neurologic melioidosis in an imported pigtail macaque (Macaca nemestrina). Veterinary Pathology Online. 2013;50(6):1139-1144.
14. Rosadio R, Cirilo E, Manchego A, Rivera H. Respiratory syncytial and parainfluenza type 3 viruses coexisting with Pasteurella multocida and Mannheimia hemolytica in acute pneumonias of neonatal alpacas. Small Ruminant Research. 2011;97(1):110-116.
15. Sommanustweechai A, Kasantikul T, Somsa W, Wongratanacheewin S, Sermswan RW, Kongmakee P, et al. Environmental management procedures following fatal melioidosis in a captive chimpanzee (Pan troglodytes). Journal of Zoo and Wildlife Medicine. 2013;44(2):475-479.
16. Tonpitak W, Sornklien C, Chawanit M, Pavasutthipaisit S, Wuthiekanun V, Hantrakun V, et al. Fatal melioidosis in goats in Bangkok, Thailand. The American journal of tropical medicine and hygiene. 2014;91(2):287-290.
17. Zehnder AM, Hawkins MG, Koski MA, Lifland B, Byrne BA, Swanson AA, et al. Burkholderia pseudomallei isolates in 2 pet iguanas, California, USA. Emerging infectious diseases. 2014;20(2):304.