WSC 2022-2023
Conference 19
Case I:
Signalment:
10-year-old intact male French Bulldog
History:
A 10-year-old intact male French Bulldog presented for evaluation of a left cranial cervical mass. One month prior to presentation, the dog was seen by his family veterinarian for weight loss and increased respiratory efforts. A complete blood count and serum biochemical profile were unremarkable. Thoracic radiographs revealed a marked diffuse bronchointerstitial pattern that was interpreted to be pneumonia and a course of antibiotics (doxycycline) was started. The clinical signs worsened, and three weeks later the dog presented to an internal medicine service with coughing, gagging, shallow breathing, and reduced appetite. A nonmobile firm mass in the left craniolateral cervical region near the mandibular angle was palpated; a computed tomography (CT) scan revealed a heterogeneous, contrast enhancing mass that compressed the larynx medially. Cytology of a fine needle aspiration of the mass was diagnostic for neoplasia, and highly suggestive of an epithelial tumor.
On presentation, the dog was bright and alert and mildly overweight (body condition score, 3.5/5). On physical examination, the dog had laborious breathing with increased abdominal effort and increased upper respiratory sounds. The rest of the physical examination and the cardiac auscultation were unremarkable. The dog underwent a head/ thorax CT the next day. The CT revealed a diffuse nodular pattern in the lungs, consistent with pulmonary metastases. The cervical mass was interpreted as an enlarged left medial retropharyngeal lymph node, and multiple tracheobronchial, sternal, mediastinal and cervical lymph nodes were rounded. The changes were diagnosed as carcinoma metastases, origin of primary mass uncertain, and the owners elected for human euthanasia.
Gross Pathology:
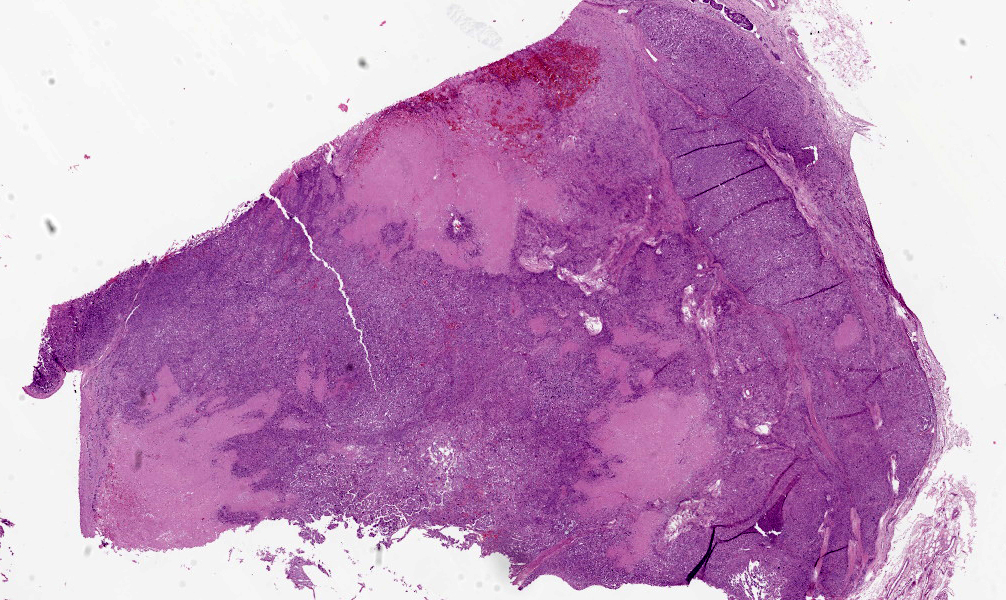
On gross post-mortem examination, intimately adherent to the outer wall of the left common carotid artery at the bifurcation into the external and internal carotid arteries, was a 3 cm in diameter firm, tan/white nodular mass that was soft to firm and mottled red/white on cut section. Another mass of similar size and appearance was identified intimately adherent to the outer wall of the aorta at the heart base. Throughout the parenchyma of the lungs were generalized firm, white to grey nodules, some of which were raised by up to 5 mm, that varied in size from 0.3 to 2.5 cm in diameter, affecting all lung lobes equally and representing approximately 75% of the lungs. Multiple bronchial, cervical, and pancreatic lymph nodes were up to 2 times enlarged and homogeneously pale tan on cut surface. Other gross abnormalities included a 2 mm in diameter, firm, tan focus within the parenchyma of the right testis.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
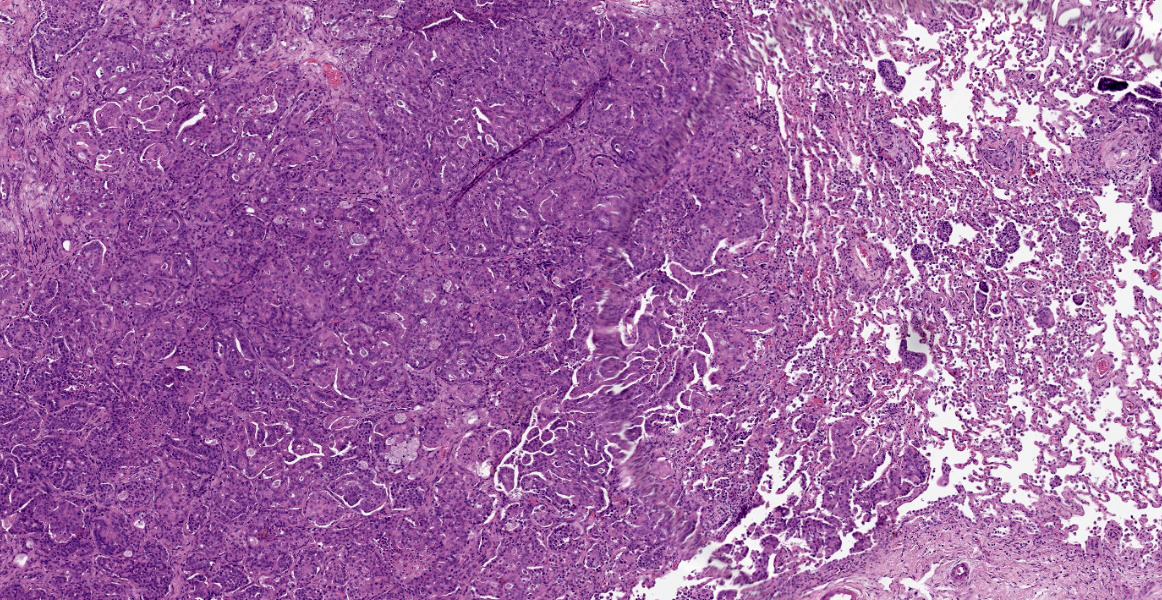
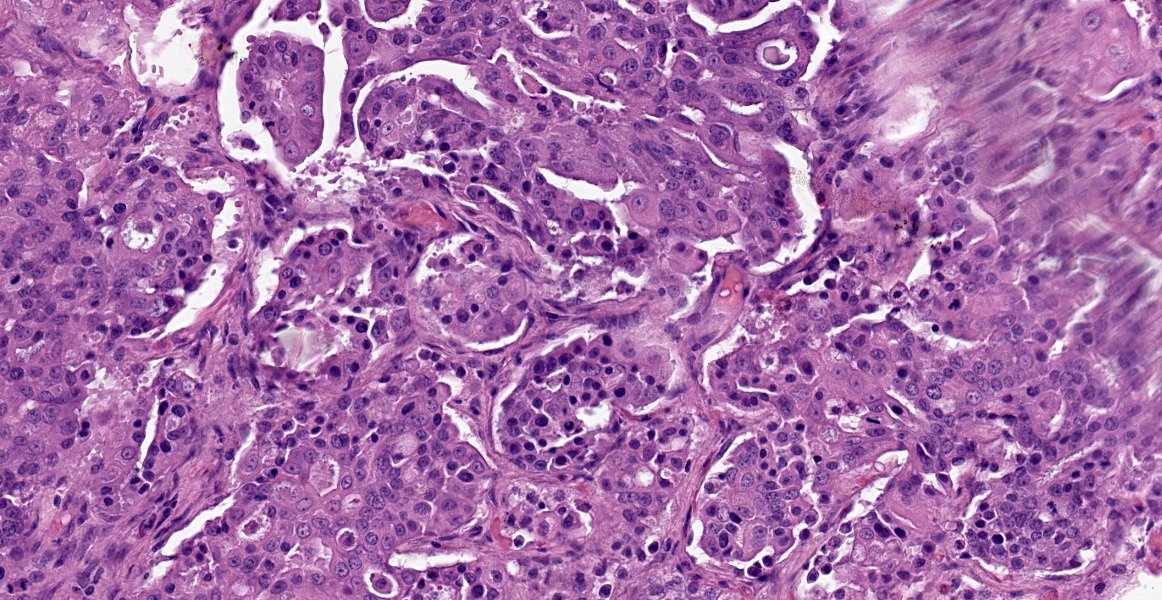
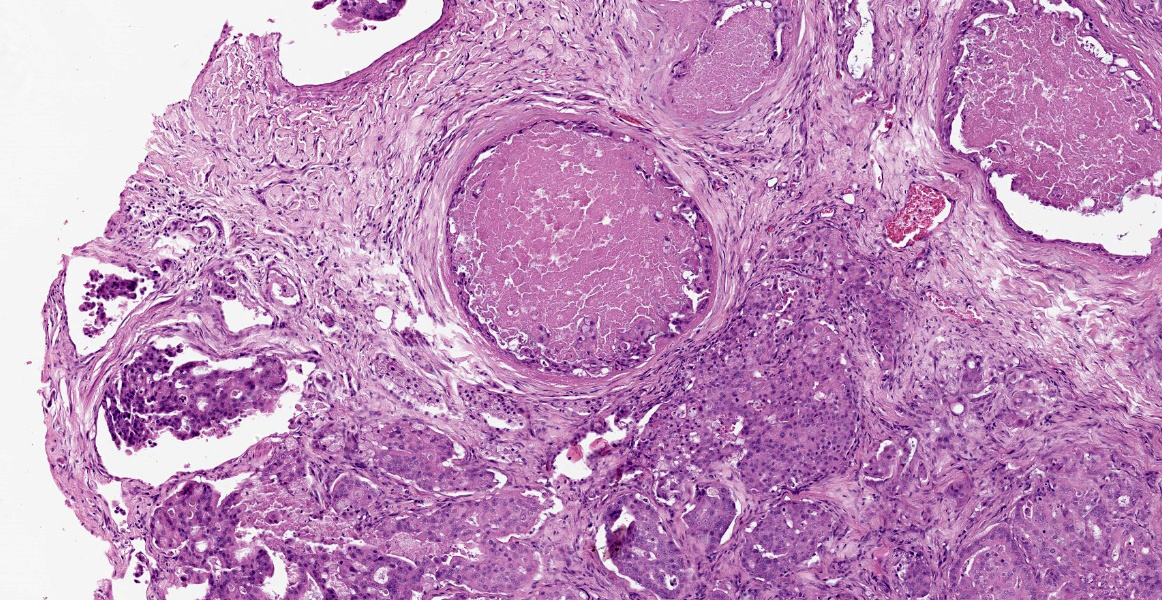
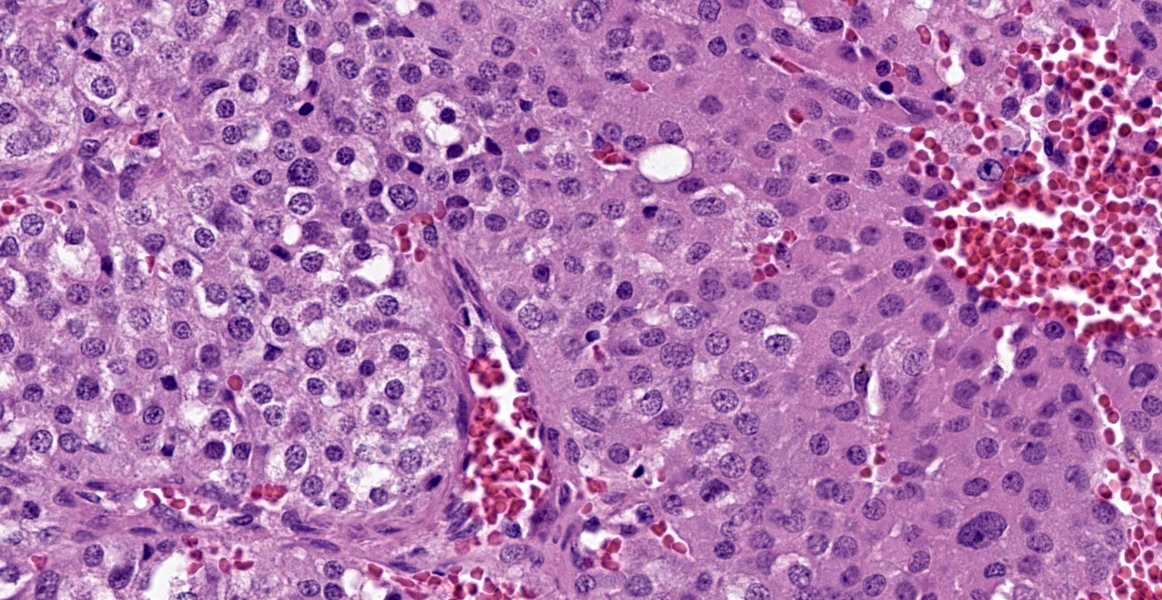
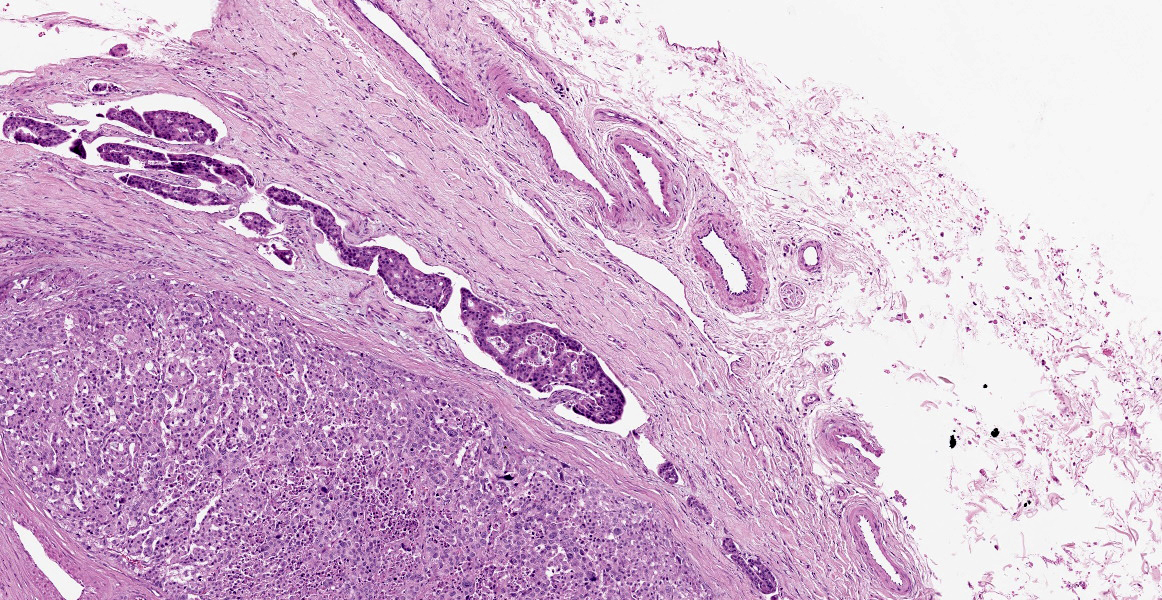
Within the connective tissue surrounding the tunica adventitia of the external and internal carotid arteries, is a partially encapsulated, focally infiltrative multilobular neoplastic mass. The mass was predominantly (50%) composed of lobules of polygonal cells separated by dense bands of connective tissue and arranged in nests and packets separated by a fine highly vascular stroma. Neoplastic cells had variably distinct borders, a moderate amount of frequently wispy granular cytoplasm, and a round to oval nucleus with coarsely granular chromatin. There was occasionally karyomegaly (up to 8 times) and rare mitotic figures.
Additionally, multifocally throughout the mass, clusters of neoplastic cells sometimes in a tubular pattern, and represented approximately 30% of the section. These regions have a different cell morphology; cuboidal neoplastic cells with distinct cell borders, moderate amounts of cytoplasm and condensed chromatin were arranged in cords and tubules, sometimes with intraluminal wispy basophilic material. In these areas, there were 11 mitotic figures per 10 high power fields (HPF). The remainder of the mass (20%) was composed of coalescing areas of necrosis, characterized by loss of tissue architecture and replacement by eosinophilic cellular debris. The aortic mass had a similar mixed morphological appearance, but with larger areas (70%) of necrosis.
Within the pulmonary parenchyma, compressing and replacing lung tissue, were multiple poorly circumscribed, infiltrative nodules of neoplastic epithelial cells that are arranged in cords and tubules that sometimes contained necrotic debris, supported and moderate stroma. The cells were cuboidal to columnar, sometimes ciliated, with distinct cell borders, abundant eosinophilic cytoplasm, and a round to oval nucleus with condensed chromatin. There was 2-fold anisocytosis and anisokaryosis and 14 mitotic figures per 10 HPF. Neoplastic cells similar to the ones described in the lung were also found effacing bronchial and pancreatic lymph nodes, and within lumen of blood and/or lymphatic vessels surrounding lungs, aorta, pancreas, and adrenal glands.
Other histological abnormalities included an interstitial cell tumor in the right testis. The cervical lymph nodes were hyperplastic and draining hemorrhage, but no neoplastic cells were present.
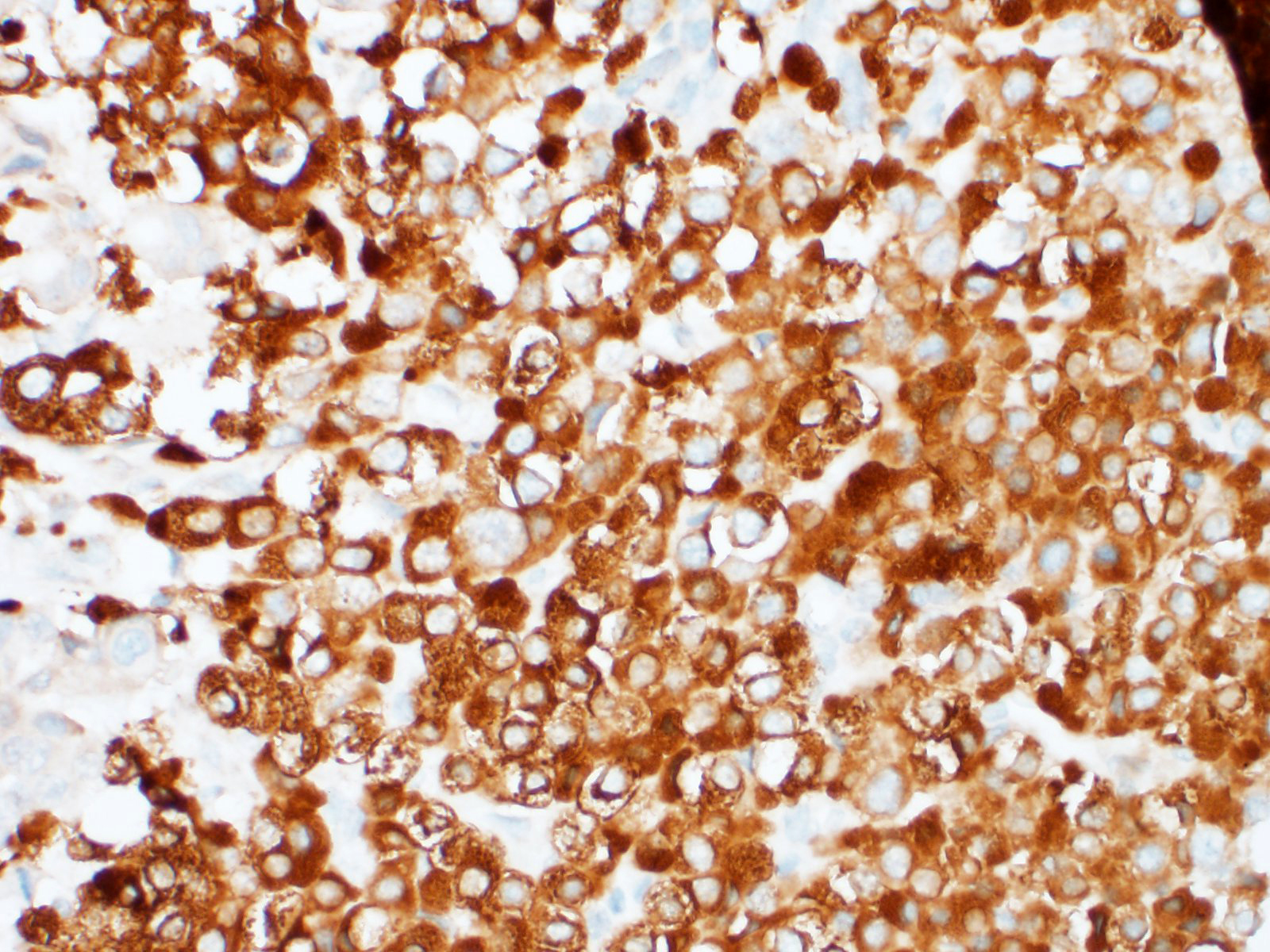
Representative sections from the carotid body tumor, the aortic body tumor, a pulmonary adenocarcinoma nodule, and a bronchial lymph node with metastasis were stained for chromogranin A (rabbit polyclonal, dilution 1:400, Dako, Glostrup, Denmark; labels approximately 65% of canine chemodectomas4,9,21), synaptophysin (rabbit monoclonal, ready to use, Ventana Medical Systems, Tucson, USA; labels 65% of canine chemodectomas4), pan-cytokeratin (mouse monoclonal, clone AE1/AE3, dilution 1:100, Dako, Glostrup, Denmark; labels 100% of canine primary pulmonary epithelial tumors [9]), and thyroid transcription factor-1 (TTF-1) (mouse monoclonal, clone 8G7G3/1, dilution 1:50, Dako, Glostrup, Denmark; labels 85% of canine primary pulmonary tumours2). External positive controls for IHC were pancreas, thyroid gland, and parathyroid gland for chromogranin A; pancreas, brain, adrenal gland and thyroid gland for synaptophysin; lung, liver, kidney, and small intestine for pan-cytokeratin; and thyroid gland for TTF-1. Slides incubated with non-immune rabbit serum or with antibody diluent were used as negative controls.
The nests of polygonal neoplastic cells showed intense diffuse cytoplasmic immunoreactivity for chromogranin A and synaptophysin in the carotid body and aortic body tumors, while tubule-forming cuboidal neoplastic cells in the chemodectomas, and pulmonary and lymph node neoplastic cells were negative. The neoplastic epithelial cells within the lung and bronchial lymph node, and the tubule-forming cuboidal neoplastic cells in the carotid body and aortic body tumors showed weak to moderate diffuse cytoplasmic and weak to intense membranous immunoreactivity for pan-cytokeratin, while the nests of polygonal cells in the chemodectomas were negative. Immunoreactivity for TTF-1 was negative in all neoplastic cells examined; intense nuclear staining was observed in occasional type II pneumocytes (internal positive control).
Contributor’s Morphologic Diagnoses:
Carotid body tumor
Aortic body tumor
Pulmonary adenocarcinoma with metastases to lung, carotid and aortic body tumors, and bronchial and pancreatic lymph nodes
Contributor’s Comment:
Based on the locations of the cervical and aortic masses, a preliminary diagnosis of carotid body and aortic body tumors with metastases to the lungs was made; representative sections of the masses and internal organs were fixed in 10% neutral buffered formalin and processed for histopathological examination. It is also worth noting that the dog is a brachycephalic breed (French bulldog), and chemodectomas have been shown to occur at a higher frequency in brachycephalic breeds such as Boxers, Bulldogs, and Boston Terriers, predominantly in middle-aged to older males.10,11,16,22 Aortic body tumors are diagnosed 4 to 12 times more often than carotid body tumors in dogs, and multiple chemodectomas are not uncommon, with 14-22% of dogs with carotid body tumors having a concomitant aortic body tumour.10,11,12, 16,22
The pulmonary masses were diagnosed as a primary pulmonary adenocarcinoma with metastases to bronchial and pancreatic lymph nodes. Given the two histologically distinct areas within the carotid body and aortic body tumors, immunohistochemistry (IHC) was done to further characterize the neoplasm. Representative sections from the carotid body tumor, the aortic body tumor, a pulmonary adenocarcinoma nodule, and a bronchial lymph node with metastasis were stained for chromogranin A, synaptophysin, pan- cytokeratin, and thyroid transcription factor-1 (TTF-1). (See microscopic description for IHC results). The IHC findings, together with the gross observations, were interpreted as a primary pulmonary acinar adenocarcinoma with metastases to carotid and aortic body tumors, and bronchial and pancreatic lymph nodes. It should be noted, however, that a primary carcinoma of unknown origin with metastases to lung and other tissues could not be ruled out.
Tumor-to-tumor metastasis is a rare phenomenon, with only about 100 human cases reported in the English literature.14 To the best of our knowledge, this has only been reported once in a domestic species; a dog with a mammary gland tumor that metastasized to a right auricular haemangiosarcoma.13 Tumor-to-tumor metastasis should not be confused with a “collision tumor”. The term “collision tumor” refers to two histologically distinct, proximally coexistent, but independent tumors. Criteria have been suggested to define a tumor-to-tumor metastasis, which include the presence of more than one primary tumor, and that the metastatic (or donor) neoplasm is a true metastasis rather than contiguous growth of two tumors (i.e., collision tumor) or embolization of tumor cells without established growth.7
In humans, the most frequent donors are lung and breast carcinomas, while the most frequent recipients are renal cell carcinomas, sarcomas, and meningiomas.17 In general, recipient tumors are slow growing, well vascularized, and can vary from benign to highly malignant. Pulmonary carcinomas have been reported to metastasize to several different types of tumours,6 with most reported cases metastasizing to renal cell carcinomas19 and intracranial meningiomas.3 There are two reports of tumor metastasis to chemodectomas; one intracranial chemodectoma with metastasis from an esophageal carcinoma15, and a carotid body tumor with metastasis from a poorly-differentiated pulmonary carcinoma.6
Concurrent primary neoplasms are not infrequent in dogs with chemodectomas. An investigation into 357 cases of dogs with chemodectomas found that 38% had at least one other microscopically confirmed primary tumour.10 The most frequent second primary tumors were thyroid carcinomas and interstitial cell tumors. Pulmonary carcinoma, seen in 6 dogs, was the 11th most frequent second primary tumor. There are several possible reasons for the co-occurrence of chemodectomas and second primary tumors. Chemodectomas metastasize infrequently (12-22% of cases), are usually slow growing, non-functional and generally found in older dogs where they can act as space-occupying lesions.10,16 Therefore, dogs diagnosed with chemodectomas simply have an extended time in which to develop a second primary tumor. There could also be a genetic component, with dogs genetically predisposed to chemodectomas also being at higher risk for other tumors. Indeed, bulldog-related breeds had a higher number of second primary tumors (47%) compared to all other breeds (32%).10
Contributing Institution:
University of Guelph
Guelph, Ontario Canada
JPC Diagnosis:
- Lung: Pulmonary adenocarcinoma.
- Fibrovascular tissue (presumed mediastinum) (2): Neuroendocrine carcinoma and metastatic pulmonary adenocarcinoma.
JPC Comment:
This week’s moderator, Colonel (Retired) Jo Lynne Raymond, and conference participants discussed whether the adenocarcinoma could be definitively diagnosed as pulmonary adenocarcinoma based on H&E. Conference participants felt the variable patterns of growth, including tubular, acinar, and lepidic patterns, and few visible cilia along the apical aspect of neoplastic cells are strongly suggestive of pulmonary origin.
Pulmonary adenocarcinomas are the most common lung tumor in dogs. Patterns of tumor growth include minimally invasive, lepidic, papillary, acinar, solid, and micropapillary, and the predominant histologic pattern can be predictive of biologic behavior.8 The first three are the most commonly reported in dogs and have the longest survival times.8 In the lepidic pattern, neoplastic cells form single-cell layer linings which trace alveolar walls, akin to a row of butterflies resting on a branch.18 Papillary growths are more cellular than the lepidic pattern, and form exophytic projections into alveolar lumens.20 In the acinar form, neoplastic cells line tubules and chords.20 Pulmonary carcinomas may also form squamous or adenosquamous patterns, with the latter being composed of at least 10% glandular and 10% squamous populations.18
In cats, tumor size is correlated with rate of metastasis8, a finding which was confirmed in a recent retrospective study by Santos et al which described gross and histologic features of pulmonary carcinomas in 39 cats.18 Tumors larger than 1.9 cm in diameter were more likely to metastasize. While metastasis to the digit has traditionally been the most well recognized in cats8, this study found metastasis to the skeletal muscle, kidneys, and parietal pleura more common than digital metastasis, reinforcing the use of the MODAL acronym (muscle, ocular, digit, aorta, lung).18 Additionally, the authors found that pulmonary carcinomas were the cause of death in only 66% of cases; cats frequently had comorbidities related to old age (lymphoma, chronic renal disease) which contributed to mortality.18
The study also evaluated P-40 and napsin-A expression in feline pulmonary carcinomas. Nuclear P-40 expression was found in all of the adenosquamous variants (with immunoreactivity in 100% of squamous cells and approximately 30% of the glandular components), which is expected for this basal cell marker expressed in cutaneous squamous cell carcinomas.18 Napsin-A, on the other hand, was non-contributory in all feline tissues.18 Napsin A is used in evaluation of human pulmonary tumors and has some utility in canine pulmonary carcinomas: another recent study showed Napsin A expression in 62 of 67 canine pulmonary adenocarcinomas.1 The specificity is relatively low, however, as it is expressed in 60% of thyroid neoplasms and renal cell carcinomas, which are important differentials to consider for any carcinoma in the lung.1
References:
- Beck J, Miller MA, Frank C, DuSold D, Ramos-Vara JA. Surfactant Protein A and Napsin A in the Immunohistochemical Characterization of Canine Pulmonary Carcinomas: Comparison with Thyroid Transcription Factor I. Vet Pathol. 2017; 54(5): 767-774.
- Bettini G, Marconato L, Morini M, Ferrari F. Thyroid transcription factor-1 immunohistochemistry: diagnostic tool and malignancy marker in canine malignant lung tumours. Vet Comp Oncol. 2009; 7: 28-37.
- Bhargava P, McGrail KM, Manz HJ, Baidas S. Lung carcinoma presenting as metastasis to intracranial meningioma: case report and review of the literature. Am J Clin Oncol. 1999; 22: 199-202.
- Brown PJ, Rema A, Gartner F. Immunohistochemical characteristics of canine aortic and carotid body tumours. J Vet Med A Physiol Pathol Clin Med. 2003; 50: 140-144.
- Burgess HJ, Kerr ME. Cytokeratin and vimentin co-expression in 21 canine primary pulmonary epithelial neoplasms. J Vet Diagn Invest. 2009; 21: 815-20.
- Bury Y, Green R, Jain M, Moor J. Metastasis of carcinoma of the lung to a carotid body paraganglioma. BMJ Case Rep. 2012.
- Campbell LV Jr, Gilbert E, Chamberlain CR Jr., Watne AL. Metastases of cancer to cancer. Cancer. 1968; 22: 635-643.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier. 2017; 495-496.
- Doss JC, Grone A, Capen CC, Rosol, TJ. Immunohistochemical localization of chromogranin A in endocrine tissues and endocrine tumors of dogs. Vet Pathol. 1998; 35: 312-5.
- Hayes HM, Sass B. Chemoreceptor neoplasia: a study of the epidemiological features of 357 canine cases. Zentralbl Veterinarmed A. 1988; 35: 401-408.
- Hayes HM. An hypothesis for the aetiology of canine chemoreceptor system neoplasms, based upon an epidemiological study of 73 cases among hospital patients. J Small Anim Pract. 1975; 16: 337-43.
- Hayes HM Jr., Fraumeni JF Jr. Chemodectomas in dogs: epidemiologic comparisons with man. J Natl Cancer Inst. 1974; 52, 1455-1458.
- Hilbe M, Hauser B, Zlinszky K, Ehrensperger F. Haemangiosarcoma with a metastasis of a malignant mixed mammary gland tumour in a dog. J Vet Med A Physiol Pathol Clin Med. 2002; 49: 443-444.
- Lee T, Cha YJ, Ahn S, Han J, Shim YM. A Rare Case of Tumor-to- Tumor Metastasis of Thyroid Papillary Carcinoma within a Pulmonary Adenocarcinoma. J Pathol Transl Med. 2015; 49: 78-80.
- Lu JQ, Khalil M, Hu W, Sutherland GR, Clark AW. Tumor-to-tumor metastasis: esophageal carcinoma metastatic to an intracranial paraganglioma. J Neurosurg. 2009; 110: 744-8.
- Patnaik AK, Liu SK, Hurvitz AI, McClelland AJ. Canine chemodectoma (extra-adrenal paragangliomas) - a comparative study. J Small Anim Pract. 1975; 16: 785-801.
- Petraki C, Vaslamatzis M, Argyrakos T, et al. Tumor to tumor metastasis: report of two cases and review of the literature. Int J Surg Pathol. 2003; 11: 127-35.
- Santos IR, Raiter J, Lamego EC, et al. Feline pulmonary carcinoma: Gross, histological, metastatic, and immunohistochemical aspects. Vet Pathol. 2023; 60(1):8-20.
- Sella A, Ro JY. Renal cell cancer: best recipient of tumor-to-tumor metastasis. Urology. 1987: 30; 35-8.
- Wilson DW. Tumors of the Respiratory Tract. In: Meuten DJ, ed. Tumors of Domestic Animals. Ames, IO: Wiley Blackwell. 2017; 487-490.
- Yamamoto S, Fukushima R, Hirakawa A, Abe M, Kobayashi M, Machida N. Histopathological and immunohistochemical evaluation of malignant potential in canine aortic body tumours. J Comp Pathol. 2013; 149: 182-191.
- Yates WD, Lester SJ, Mills JH. Chemoreceptor tumors diagnosed at the Western College of Veterinary Medicine 1967-1979. Can Vet J. 1980; 21: 124-129.