Signalment:
Dog 1: 7-year-old female spayed Cavalier King Charles Spaniel (Canis familiaris).
Dog 2: 12-year-old female spayed miniature Poodle (Canis familiaris). Four dogs presented to our referral hospital over a seven-week period with clinical signs of severe liver disease (including one or more of: inappetence, lethargy, vomiting, diarrhoea, polyuria and polydipsia). All four dogs had a common history of consuming a cooked commercial camel meat and sweet potato diet as a novel protein for management of suspected allergic skin disease. Two of these dogs subsequently deteriorated despite supportive therapy and were euthanized. Dog 1 was submitted for a full necropsy examination whereas surgical biopsy specimens of liver and pancreas were collected from dog 2 two days prior to euthanasia.
Gross Description:
Histopathologic Description:
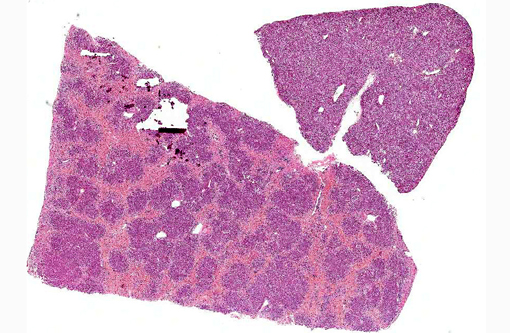
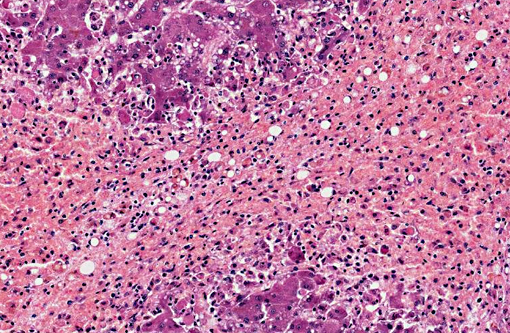
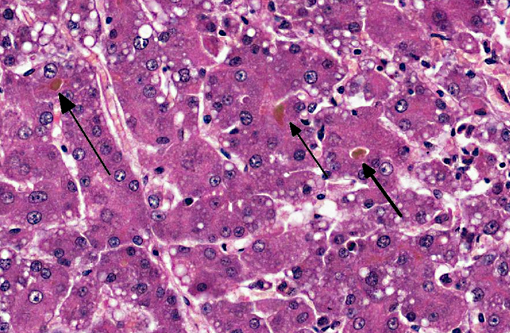
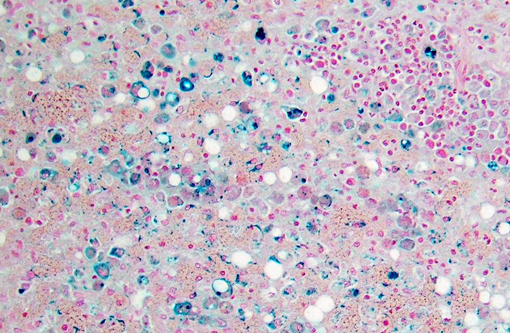
Dog 1, liver (larger section of tissue): This section demonstrates extensive disruption of the hepatic architecture by diffuse, centrilobular to massive zonal hepatocyte loss and haemorrhage. Numerous macrophages containing erythrocytes or a fine, golden to coarse dark brown granular cytoplasmic pigment are scattered extensively throughout the areas of hepatocyte loss and haemorrhage, and to a lesser extent the intact hepatic parenchyma. The majority of the cytoplasmic granules stain blue with a Perls Prussian Blue (haemosiderin). Infiltrating portal triads and scattered throughout the areas of haemorrhage, are frequent clusters of lymphocytes and plasma cells accompanied by fewer neutrophils and macrophages. Within the residual clusters of hepatocytes there are often plugs of bile within distended canaliculi. Bile ducts are often distended by variably cellular casts. The remaining hepatocytes demonstrate moderate anisocytosis, often associated with cytoplasmic macro- and microvesicular vacuolation. There are also scattered individually necrotic hepatocytes. Subcapsular and portal lymphatics are often ectatic. Histochemical staining with Martius Scarlett Blue highlighted the presence of a mild increase in fibrous connective tissue surrounding the portal triads. A thin strand of fibrous connective tissue also dissects between individual hepatocytes in the centrilobular regions.
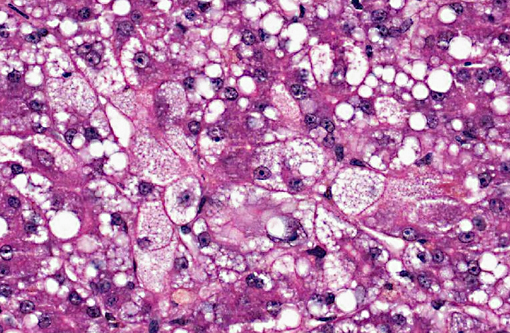
Dog 2, liver (smaller section of tissue): The architecture of the liver is disrupted by mild to moderate, diffuse bridging fibrosis which connects central veins. Associated with the fibrosis is a moderate, multifocal, mixed infiltrate of haemosiderophages (confirmed with a Perls stain for iron), lymphocytes and plasma cells and frequent dilated lymphatics. Portal triads are also expanded by mild to moderate fibrosis, mild biliary hyperplasia and an infiltrate of modest numbers of haemosiderophages, lymphocytes and occasional neutrophils. Associated with the centrilobular and portal fibrosis, there is moderate variation in hepatic lobule size, which is attributable to a combination of hepatocyte degeneration, atrophy and loss particularly surrounding the central veins. Remaining hepatocytes also demonstrate moderate to marked anisocytosis associated with cytoplasmic macrovesicular and microvesicular vacuolation. Occasional hepatocytes are binucleate and some have prominent, multiple nucleoli. There is also occasional single cell necrosis of hepatocytes. The undulating appearance of the capsular surface is attributable to the predominately centrilobular hepatocyte loss, lobular collapse and fibrosis.Â
Pancreas (sections not included): Examination of the pancreas of both dogs reveals multifocal to coalescing foci of pancreatic fibrosis with degeneration, atrophy and loss of adjacent exocrine pancreatic acini. These changes are accompanied by variably extensive foci of peripancreatic fat necrosis and a neutrophilic infiltrate; minimal and focal in dog 1 but severe and reasonably extensive in dog 2.Â
Morphologic Diagnosis:
Dog 1 (larger tissue section):
- Liver:
- Severe, diffuse, subacute to chronic, centrilobular to massive hepatic necrosis and loss, with marked haemorrhage and haemosiderosis.
- Mild to moderate, multifocal, chronic, mixed hepatitis.
- Mild, periportal to dissecting, subacute, hepatic fibrosis.
- Mild, multifocal, subacute to chronic vacuolar hepatopathy.
Dog 2: (smaller tissue section)
- Liver:
- Mild to moderate, chronic, centrilobular to bridging fibrosis, haemorrhage and haemosiderosis.
- Moderate, diffuse subacute to chronic vacuolar hepatopathy.
- Mild to moderate, subacute to chronic, multifocal lymphoplasmacytic hepatitis.Â
Lab Results:
Serum biochemistry:
Dog 1:
| Day 1 | Day 13 | Day 18 | Day 19 | Reference Range | |
| Lipase | 1500 | - | - | 2500 | 13-200 U/L |
| Amylase | 2200 | - | - | 3500 | 65-1140 U/L |
| ALT | 1100 | 1500 | - | 1455 | 21-142 U/L |
| ALP | 550 | 1000 | - | 900 | 20-184 U/L |
| AST | N | 290 | - | - | 10-60 U/L |
| GGT | 13 | 30 | - | - | |
| Bilirubin | N | 36 | - | 181 | 0-8 umol/L |
| Cholesterol | 9.6 | 10 | - | 10 | 3.3-6.9 mmol/L |
| Urea | N | - | - | 1.9 | 3.6-10.0 mmol/L |
| PT | - | - | 19.4 | - | 5.1-7.9 secs |
| APTT | - | - | 24.8 | - | 8.6-12.9 secs |
(-) = not performed
Dog 2:
| Day 1 | Day 5 | Day 6 | Day 7 | Day 8 | Reference Range | |
| ALT | 3120 | 3200 | - | 1960 | 1460 | 21-142 U/L |
| ALP | 136 | 633 | - | 561 | 531 | 20-184 U/L |
| AST | 926 | 956 | - | 687 | 577 | 10-60 U/L |
| Bilirubin | 8 | 134 | - | 144 | 123 | 0-8 umol/L |
| Cholesterol | 5.3 | 6.0 | - | 4.6 | 4.1 | 3.3-6.9 mmol/L |
| Urea | 4.1 | 4.5 | - | 2.3 | 3.7 | 3.6-10.0 mmol/L |
| PT | - | - | 18.5 | - | - | 5.1-7.9 secs |
(-) = not performed
Toxicology: Indospicine was subsequently detected in the serum or plasma of both dogs as well as in samples of liver and skeletal muscle of dog 1. Detection of indospicine in a sample of the cooked camel meat and sweet potato diet fed to one of the dogs, as well as in samples of camel meat provided by the manufacturer, confirmed the source of the toxin. Aflatoxins B1, B2, G1 & G2 were not detected in either of these samples.Â
Indospicine was also detected in the serum of the other two dogs that developed clinical signs of hepatic disease, although these dogs subsequently recovered with supportive therapy and have remained well for the duration of follow up. In addition, indospicine was similarly detected in the serum of 15 other dogs known to have been consuming the diet. 3/15 of these dogs had increased serum ALT activity, although with the exception of one dog, none developed clinical signs of liver disease. One dog however subsequently went on to develop clinical signs of severe liver disease, despite withdrawal of the contaminated diet 5 months prior and was euthanized.Â
Condition:
Contributor Comment:
Mammals appear to have a poor ability to metabolize or excrete indospicine, and experimentally levels have been shown to persist in the tissues (including skeletal muscle) of horses, goats, cattle and rabbits for several months following cessation of Indigofera spp. intake.(6) Such indospicine residues have the potential to cause secondary toxicosis in carnivores consuming contaminated meat and severe, sometimes fatal hepatotoxicosis has been reported in dogs consuming contaminated horse and camel meat. There is, however, marked species variability in susceptibility to indospicine hepatotoxicosis with dogs being particularly sensitive.(6,7) Cattle and sheep are unaffected under normal grazing conditions; however, liver damage has been experimentally induced under conditions of high Indigofera spp. intake.(6) Horses appear resistant to the hepatotoxic effects of indospicine; however, horses grazing I. linnaei (Birdsville indigo) may suffer from a neurological disturbance known as Birdsville horse disease,(3) presumably due to co-occuring esters of 3-nitropropanoic acid.(17) There are no reports of indospicine or Indigofera-associated toxicosis in camels, although the remote location of these animals in the arid regions of Australia generally limits close observation.(6)
The reason behind the species variability and apparently unique susceptibility of dogs to the hepatotoxic effects of indospicine is similarly unknown. Indospicine is also not a predictable hepatotoxin in dogs, although the characteristic lesions of hepatocellular vacuolation, necrosis, haemorrhage and mononuclear inflammatory cell infiltration primarily seem to target the centrilobular hepatocytes. Note that the cause of the concurrent pancreatic inflammation and fibrosis seen in both of these dogs is unknown,(7) as it has not been previously reported in cases of indospicine toxicosis in animals, despite the pancreas having the highest levels of indospicine in a variety of tissues tested.(12,17) The trends seen in naturally occurring cases,(6,7) as well the results of experimental trials in dogs(12,17) show a high degree of variability in the susceptibility of individual dogs to indospicine hepatotoxicosis, with liver failure only occurring in a small proportion of dogs exposed.(6,7,12,17) These findings have led the suggestion that an idiosyncratic response to the toxin may occur, perhaps in addition to a more consistent toxic effect. The nature of the inflammatory response has also led to postulation that an immune-mediated mechanism may be involved.(16) Whatever the exact mechanism of liver injury, the available evidence suggests that indospicine is not a rapidly necrotizing hepatotoxin. Based on the results of previous experimental studies, prolonged exposure (4 days) is seemingly required for development of clinical disease, with no dogs in short term trials (dosed over four consecutive days) developing signs of liver disease.(2,7,17)
JPC Diagnosis:
1. Liver: Necrosis, centrilobular to massive, bridging, severe, with marked cholestasis and macro and microvesicular hepatocellular lipidosis.
2. Liver, hepatocytes: Macro and micronodular lipidosis with multifocal hepatocyte necrosis, and loss, mild bridging fibrosis and mild cholestasis.
Conference Comment:
Although the most striking microscopic lesions in this case are the massive hepatocellular necrosis and loss along with cholestasis and lipidosis, there is also mild bridging fibrosis in dog 2, which was demonstrated with a Massons stain. This subtle suggestion of chronicity supports the contributors supposition that indospicine toxicity is likely not a peracute/acute hepatotoxin. Given that indisopicine containing species such as Indigofera are not generally found in North America, most conference participants formulated a list of alternative hepatoxins that could potentially induce similar lesions dogs, including aflatoxicosis, pyrrolizidine alkaloid toxicosis, sago palm toxicity, blue-green algae toxicity, copper toxicosis, iron overload and amanita toxicity.
References:
2. Bain PJ. Liver. In: Latimer KS, ed. Duncan and Prasses Veterinary Laboratory Medicine Clinical Pathology. 5th ed. Ames, IA: John Wiley & Sons; 2011:134-136, 211-225, 274-277, 416.
3. Bell AT, Everist SL. Indiogofera enneaphylla: a plant toxic to horses (Birdsville disease). Aust Vet J. 1951;27:185-188.
4. Britten EJ, Palofox AL, Frodyma MM, Lynd FT. Level of 3-nitropropanoic acid in relation to toxicity of Indigofera spicata in chicks. Crop Sci. 1963;3:415-416.Â
5. Christie GS, Wilson M, Hegarty MP. Effects on the liver in the rat of ingestion of Indigofera spicata, a legume containing an inhibitor of arginine metabolism. J Pathol. 1975;117:195-205.
6. FitzGerald LM, Fletcher MT, Paul AEH, Mansfield CS, OHara A. Hepatotoxicosis in dogs consuming a diet contaminated with indospicine. Aust Vet J. 2011;89: 95-100.Â
7. Hegarty MP, Kelly WR, McEwan D, Williams OJ, Cameron R. Hepatotoxicity to dogs of horse meat contaminated with indospicine. Aust Vet J. 1988;65:337-340.
8. Hegarty MP, Pound AW. Indospicine, a hepatotoxic amino acid from Indigofera spicata: isolation, structure, and biological studies. Aust J Biol Sci. 1970;23:831-842.
9. Hegarty MP, Pound AW. Indospicine, a new hepatotoxic amino acid from Indigofera spicata. Nature . 1968;217:354-355.
10. Hutton EM, Windrum GM, Kratzing CC. Studies on the toxicity of Indigofera endecaphylla: II. Toxicity for mice. J Nutr. 1958;65:429-440.
11. Hutton EM, Windrum GM, Kratzing CC. Studies on the toxicity of Indigofera endecaphylla: I. Toxicity for rabbits. J Nutr. 1958;64:321-337.Â
12. Kelly WR, Young MP, Hegarty MP, Simpson GD. The hepatotoxicity of indospicine in dogs. In: James LF, Keeler RF, Bailey EM, Cheeke PR, Hegarty MP, eds. Poisonous Plants. Ames, Iowa: Iowa State University Press; 1992:126-130.
13. Madsen NP, Hegarty MP. Inhibition of rat liver homogenate arginase activity in vitro by the hepatotoxic amino acid indospicine. Biochem Pharmacol. 1970;19:2391-2393.
14. Pearn J. An experimental study of the embryopathic effects of indospicine: with particular reference to the production of cleft palate. MD thesis, University of Queensland, St. Lucia, Brisbane, 1967. Cited in: Young MP: Investigation of the toxicity of horsemeat due to contamination by indospicine. PhD thesis, School of Veterinary Science, University of Queensland, Brisbane; 1992:28-30.
15. Pollitt S, Hegarty MP, Pass MA. Analysis of the amino scid indospicine in biological samples by high performance liquid chromatography. Nat Toxins. 1999;7:233-240.Â
16. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie, MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:376-378.
17. Young MP. Investigation of the toxicity of horsemeat due to contamination by indospicine. PhD thesis, School of Veterinary Science, University of Queensland, Brisbane, 1992.