WSC 2022-2023
Conference 18
Case IV:
Signalment:
12-year-old male striped hyena (Hyaena hyaena)
History:
A firm, non-painful, approximately 3-cm-diameter subcutaneous mass was observed elevating skin over the dorsal cervical region. Skin was intact and freely moveable over the surface of the mass. The mass was monitored for a period of several months with no obvious changes. A diagnosis was first made from punch biopsies, following which the entire mass was excised.
Gross Pathology:
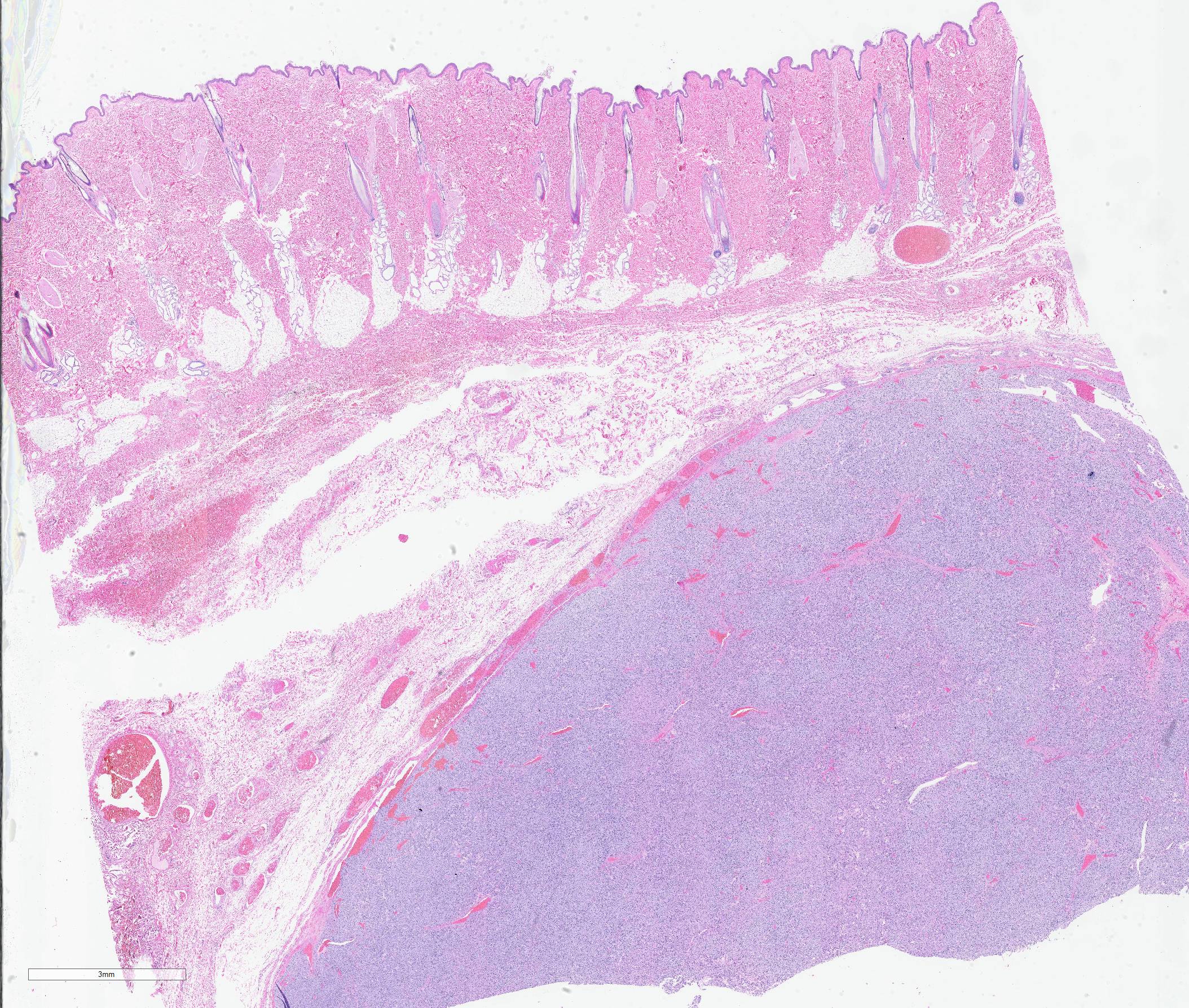
The specimen submitted was an approximately 7 x 7 cm elliptical section of haired skin, extending to panniculus musculature at its deep margin. The skin surface was centrally elevated and surrounding tissue compressed by a well-demarcated, firm, approximately 3-cm-diameter, multilobular mass. On cut surfaces, the mass was homogeneously white to pale pink, with lobules separated by septa of dense connective tissue.
Laboratory Results:
Serum biochemistry:
Within normal limits
CBC:
WBC 13.2x103/µL; 1% band neutrophils, 73% segmented neutrophils, 21% lymphocytes, 3% monocytes
HCT: 42.7%
Microscopic Description:
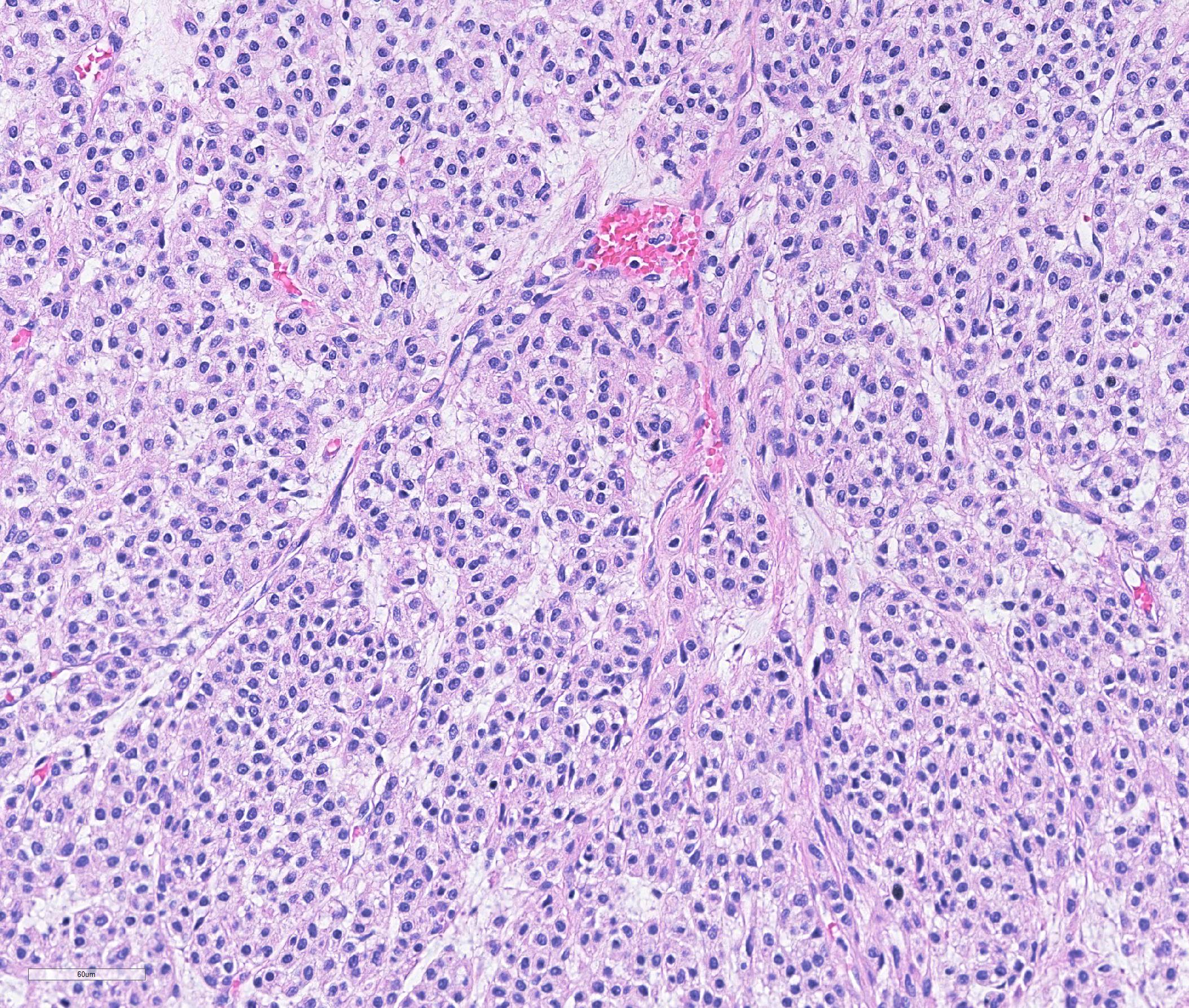
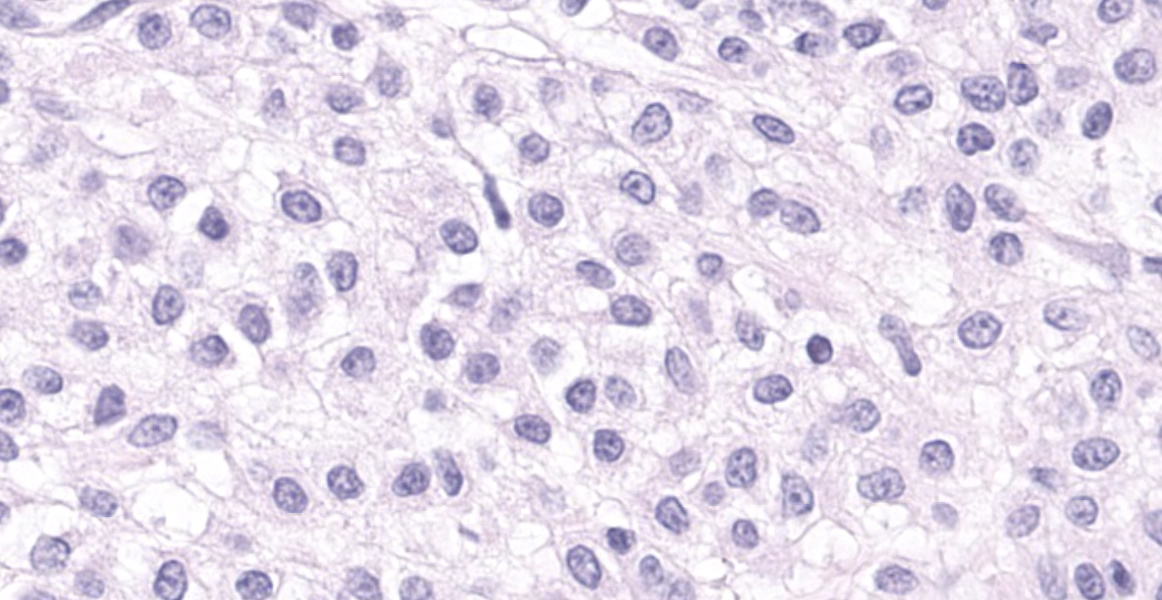
Haired skin: A densely cellular, multilobular, pseudoencapsulated, expansile neoplasm expands the subcutis, elevating the overlying dermis and compressing adjacent tissues. Neoplastic lobules sometimes abut or protrude into large vascular spaces, especially at the periphery, but remain separated from lumina by a thin layer of endothelium. The neoplastic cell population consists of round to polygonal to sometimes spindloid cells arranged in solid sheets, cords, or packets, supported by a fine fibrovascular stroma. The cells have moderate amounts of pale eosinophilic, vacuolated cytoplasm and indistinct cytoplasmic borders. Nuclei are centralized and round to ovoid, sometimes reniform or indented, with vesicular or dispersed chromatin. Anisocytosis and anisokaryosis are moderate. Mitotic figures are infrequent, with 4 mitotic figures seen in ten 400x fields in the most mitotically active regions. Scattered individual tumor cells are hypereosinophilic and have condensed nuclear material, and there are a few small foci of necrosis and associated hemorrhage within the mass. In a few areas there are aggregates of fibrin and neutrophils adjacent to or partially occluding vascular lumina (thrombosis). In some sections, there is a broad zone of necrosis and regional hemorrhage with moderate numbers of hemosiderin- and hematoidin-laden macrophages (previous biopsy site).
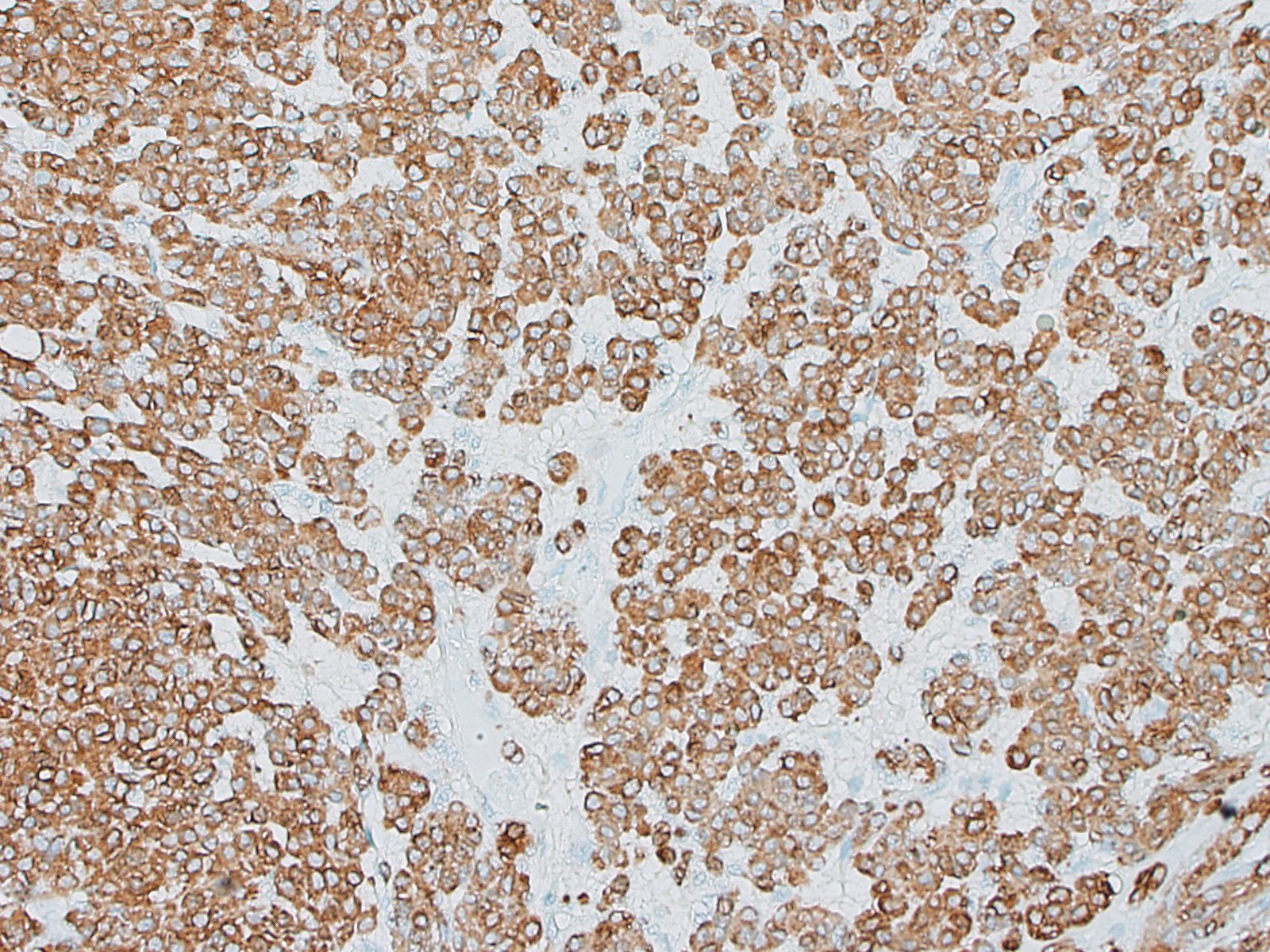
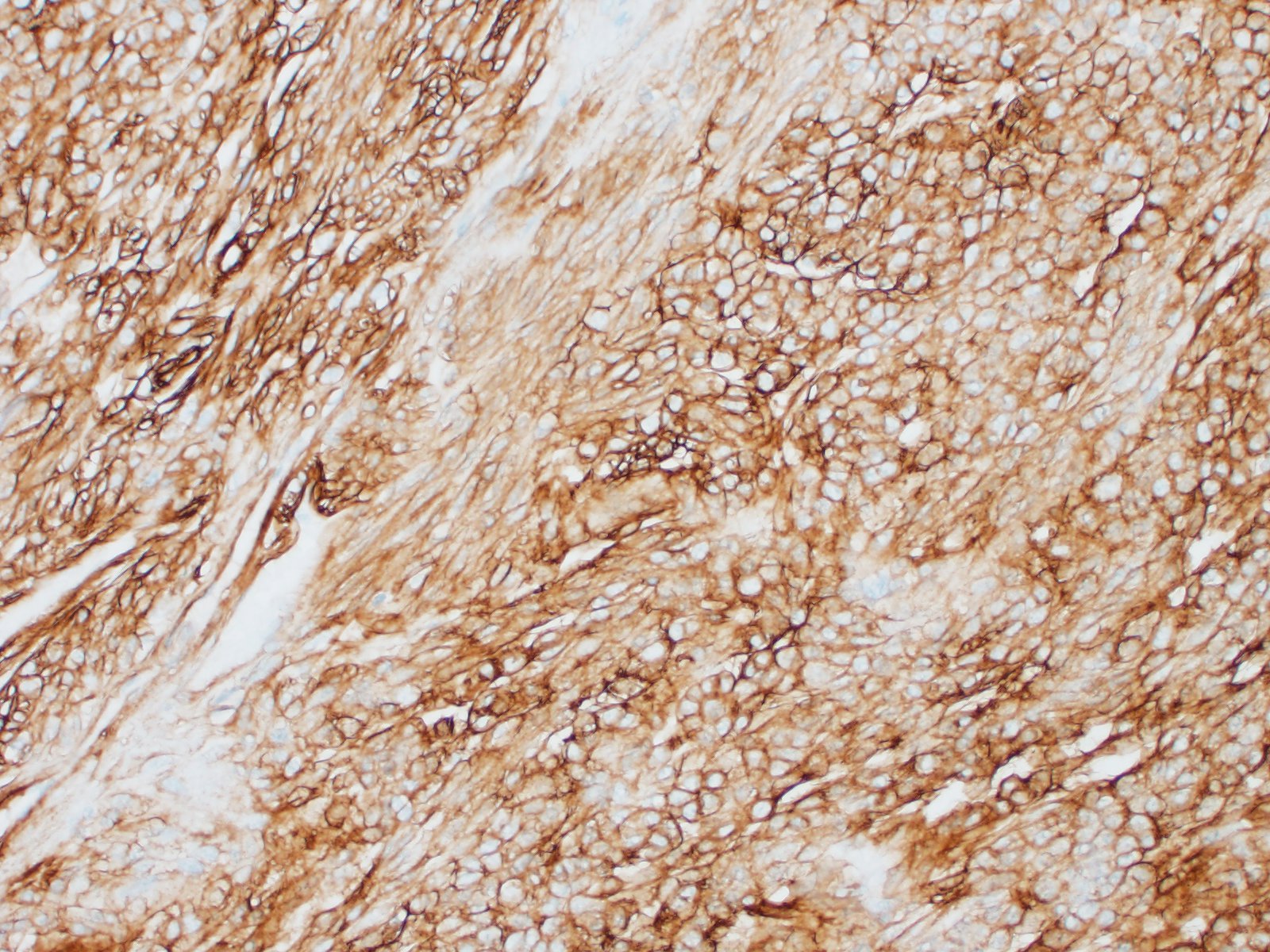
Special stains and immunohistochemistry: Fine basement membranes surrounding individual tumor cells or small clusters of cells are immunoreactive for laminin and are also clearly highlighted with a Periodic acid-Schiff (PAS) stain. Neoplastic cells exhibit strong, punctate, cytoplasmic immunoreactivity for vimentin and α-smooth muscle actin. Immunohistochemical staining for desmin and pancytokeratin is negative.
Contributor’s Morphologic Diagnoses:
Subcutis: glomus tumor
Contributor’s Comment:
Glomus bodies are specialized arteriovenous shunts that function in thermoregulation by modifying capillary perfusion.8 They are found most frequently in skin of the distal extremities, particularly in digital skin and subungual regions. In glomus bodies, the branching pre-glomic arterioles blend into arteriovenous anastomoses (Sucquet-Hoyer canals) with surrounding modified smooth muscle cells termed “glomus cells.” They are richly invested with small nerves and blood vessels, and are circumscribed by dense, lamellar collagen.
Glomus tumors arise from the modified smooth muscle cells of the glomus body. They are uncommon but well-recognized in humans, where they occur most often as solitary nodules in subungual sites and in the dermis or subcutis of the digits and distal extremities.8 They occur rarely in other sites, including visceral organs and bone.8 Glomus tumors are often reported to be exquisitely painful, likely as a consequence of their rich peripheral nerve supply.8
Histologically, glomus tumors tend to be well-circumscribed and consist of densely cellular sheets and cords of epithelioid cells surrounding and abutting vascular structures, with fine basement membranes surrounding individual cells or clusters of cells. Several histologic variants are described in humans, including solid tumors, angiomatous glomus tumors (glomangiomas), myxoid glomus tumors, and oncocytic glomus tumors.7 Malignant glomus tumors (glomangiosarcomas) are exceedingly rare. Even in glomus tumors with focal or regional cytologic features of malignancy, invasive behavior and distant metastases are uncommon.7-9
Using immunohistochemistry, the neoplastic cells are routinely positive for a-smooth muscle actin and vimentin. Desmin immunoreactivity is variable, and pancytokeratin immunoreactivity is uniformly negative. Basement membranes surrounding individual cells or clusters of cells can be demonstrated by PAS stains or by positive immunoreactivity for laminin and collagen IV.
Glomus tumors are rare in veterinary species, but have been reported in dogs, cats, horses, bovids, and non-human primates.1-3,5,6,11,12,14,-16,18,20 Digital/subungual sites (or homologous regions) were affected in two dogs, a cat, and a horse.2,3,5,20 Glomus tumors of the skin of the head or neck were reported in three equine cases, two of which were diagnosed as malignant variants.2 In bovids, the two reports of glomus tumors describe neoplasms of the liver and of the urinary bladder.10,16
Proposed criteria suggesting malignancy include (1) large size (>2 cm diameter) with deep location (deep to muscular fascia); (2) presence of atypical mitotic figures; or (3) nuclear atypia with relatively high mitotic rate (>5/50 high power fields).4 Vascular involvement is not reported to be associated with malignancy. In this case, the mass was relatively large (~3 cm in diameter) but superficial. Atypical mitotic figures were not a significant feature. There was mild nuclear atypia, but a relatively high mitotic rate in some areas. Based on the criteria suggested above, this mass would warrant the (admittedly non-committal) designation of “glomus tumor of uncertain malignant potential.” Radiographs taken at the time of excisional biopsy did not demonstrate metastatic lesions, and no evidence of recurrence or metastasis has been noted in the four months following excision.
Contributing Institution:
Disease Investigations
Institute for Conservation Research
San Diego Zoo Global
PO Box 120551
San Diego, CA 92112
http://institute.sandiegozoo.org/disease-investigations
JPC Diagnosis:
Haired skin: Glomus tumor.
JPC Comment:
This case is a classic example of a glomus tumor in a novel species. Glomus tumors are typically well-demarcated neoplasms composed of densely packed round to spindleloid cells which occasionally form nests.10,17 Neoplastic cells frequently surround entrapped vascular channels, which may appear as blood-filled clefts, or may surround larger cavernous vessels (glomangiomas).10,13 As the contributor mentions, the tumor is frequently associated with vessels and nerves, which may be visible along the tumor margin.10 Differential diagnoses include Merkel cell tumors (arranged in a packet pattern), plasma cell tumors (cells are more pleiomorphic with eccentric nuclei), histocytomas (more top-heavy in distribution), other round cell neoplasms, and leiomyoma (particularly of the arrector pili muscle, or piloleiomyoma).10 Other differentials considered by the moderator and participants were hidradenoma (as some areas of packeting mimicked tubules), melanoma, and a neuroendocrine tumor. When histologic findings are equivocal, special stains and IHCs may be necessary for differentiation, as described by the contributor.
A potential source for confusion in this uncommon veterinary neoplasm are the human entities glomus jugulotympanicum paraganglioma and glomus tympanicum paraganglioma.13,19 While they contain the word glomus in the name, they are not associated with the glomus bodies in the dermis and subcutis. Rather, they are paragangliomas arising from neural-crest derived chemoreceptor cells of the paraganglia located along certain cranial nerves and within the jugular foramen.13,19 As paragangliomas, neoplastic cells are positive for synapthophysin and chromogranin A on immunohistochemistry.19
References:
- Brounts SH, Adams SB, Vemireddi V, Holland CH. A malignant glomus tumour in the foot of a horse. Equine Vet Educ.2008;20:24-27.
- Burns RE, Pesavento PA, McElliott VR, Ortega J, Affolter VK. Glomus tumours in the skin and subcutis of three horses. Vet Dermatol. 2011;22:225-231.
- Dagli ML, Oloris SC, Xavier JG, dos Santos CF, Faustino M, Oliveira CM, Sinhorini IL, Guerra JL. Glomus tumour in the digit of a dog. J Comp Pathol.2003;128:199-202.
- Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
- Furuya Y, Uchida K, Tateyama S. A case of glomus tumor in a dog. J Vet Med Sci. 2006;68:1339-1341.
- Galofaro V, Rapisarda G, Ferrara G, Iannelli N. Glomangioma in the prepuce of a dog. Reprod Dom Anim. 2006;41:568-570.
- Goldblum JR. Soft Tissues. In: Goldblum JR, Lamps LW, McKenney JK, Myers JL, eds. Rosai and Ackerman’s Surgical Pathology, 11th Philadelphia, PA: Elsevier; 2018:1810-1914.
- Goldblum JR, Folpe AL, Weiss SW. Perivascular Tumors. In: Goldblum JR, Folpe AL, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors, 6th ed. Philadelphia, PA: Elsevier; 2014:749-765.
- Gould EW, Manivel JC, Albores-Saavedra J, Monforte H. Locally infiltrative glomus tumors and glomangiosarcomas. Cancer. 1990;65:310-318.
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Perivascular tumors. In: Skin Diseases of the Dog and Cat Clinical and Histopathological Diagnosis. 2nd Ames, IA: Blackwell Science Ltd.; 2005:759-762.
- Horiuchi N, Komagata M, Shitamura K, Chiba S, Matsumoto K, Inokuma H, Matsui T, Kobayashi Y. Glomus tumor in the liver of a cow. J Vet Med Sci. 2015;77(6):729-732.
- Hubbard GB, Wood DH. Glomangiomas in four irradiated Macaca mulatta. Vet Pathol. 1984;21:609-610.
- Miettinen M, Fetsch JF, Antonescu CR, Folpe AL, Wakely Jr PE. Smooth Muscle Tumors. In: Tumors of the Soft Tissues. Silver Spring, MD: ARP Press. 2014; 274-278.
- Park CH, Kozima D, Tsuzuki N, Ishi Y, Oyamada T. Malignant glomus tumour in a German shepherd dog. Vet Dermatol. 2009;20:127-130.
- Peters M, Grafen J, Kuhnen C, Wohlsein P. Malignant glomus tumour (glomangiosarcoma) with additional neuroendocrine differentiation in a horse. J Comp Pathol. 2016;154:309-313.
- Roperto S, Borzacchiello G, Brun R, Perillo A, Russo V, Urraro C, Roperto F: Multiple glomus tumors of the urinary bladder in a cow associated with bovine papillomavirus type 2 (BPV-2) infection. Vet Pathol.2008;45:39-42.
- Santa Cruz DJ, Gru AA. Tumors of the Skin. In: Fletcher DM, ed. Diagnostic Histopathology of Tumors. Vol 2. 5th Philadelphia, PA: Elsevier. 2021; 1814-1815.
- Shinya K, Uchida K, Nomura K, Ozaki K, Narama I, Umemura T. Glomus tumor in a dog. J Vet Med Sci.1997;59:949-950.
- Thompson LDR. Tumors of the Ear. In: Fletcher DM, ed. Diagnostic Histopathology of Tumors. Vol 2. 5th Philadelphia, PA: Elsevier. 2021; 2271.
- Uchida K, Yamaguchi R, Tateyama S. Glomus tumor in the digit of a cat. Vet Pathol.2002;39:590-592.