Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 14
January 10th, 2018
CASE I: BB364/11 (JPC 4019414).
Signalment: 9-year-old, female, spayed, Collie cross, Canis familiaris, canine.
History: This dog was referred to the Royal (Dick) School of Veterinary Studies, University of Edinburgh, with a two-month history of persistent watery diarrhea, weight loss and vomiting. The dog had been imported from Italy under the UK’s Pet Passport Scheme in 2001. On initial presentation, the dog was bright and alert but thin. The only other physical abnormality was palpable thickening of a loop of bowel in the cranial abdomen. On routine hematology there was lymphopenia and a single biochemical abnormality of a moderately increased ALT (310 IU/l, ref 21–102 IU/L). Abdominal ultrasound confirmed thickening of the jejunal wall. Exploratory celiotomy confirmed marked thickening of one third of the jejunum but found no evidence of obstruction. Biopsies were taken on two separate occasions. The first set consisted of small, full-thickness biopsies from which the initial diagnosis was made; the second set consisted of three portions originating from an 80cm resected segment of thickened jejunum. The dog recovered well and has remained asymptomatic.
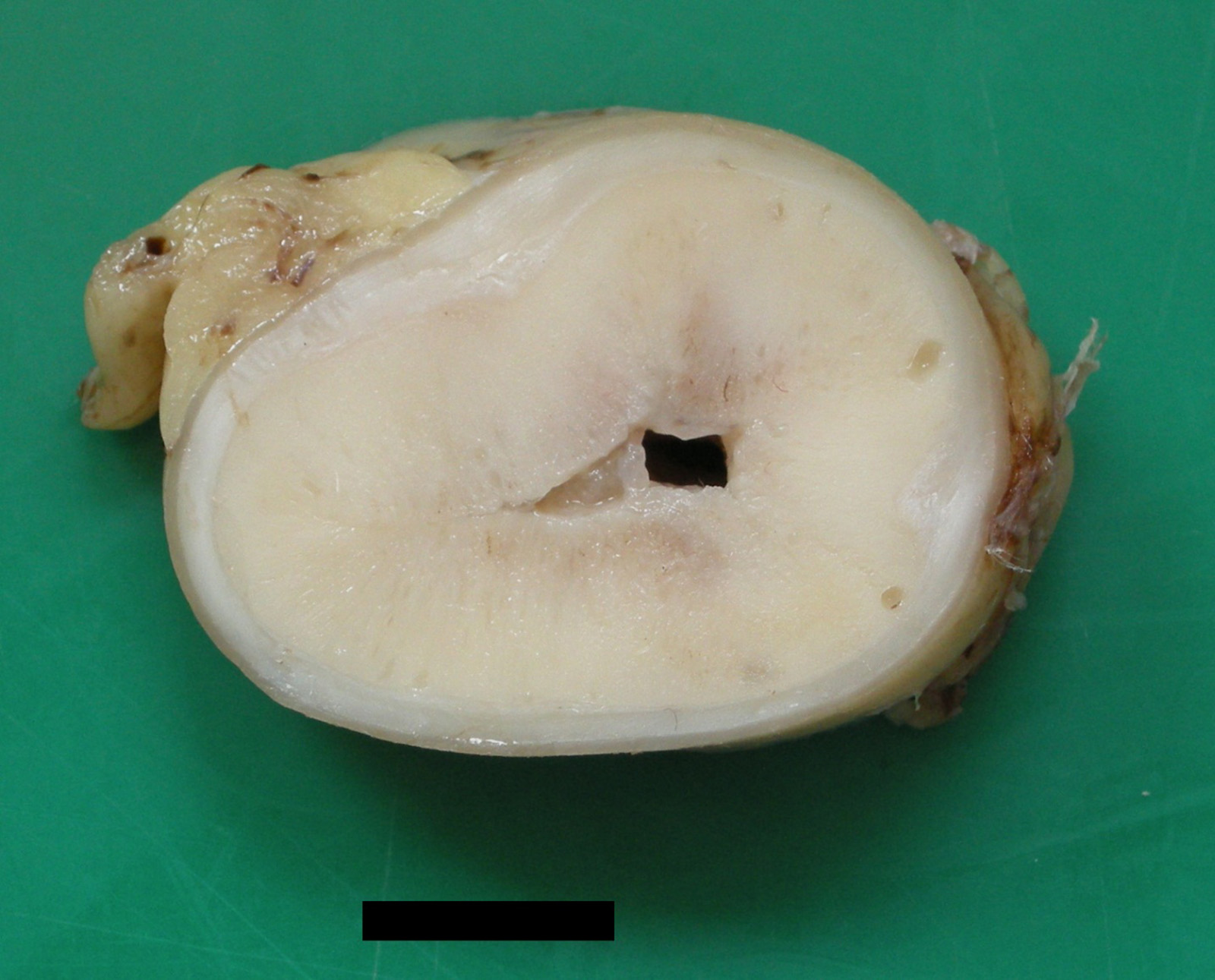
Gross Pathology: Gross lesions were most appreciable in the portions of resected jejunum taken at the second surgery. In the most severely affected segment, the wall was thickened up to 3.5cm. On transverse section, this thickening was circumferential and consisted of firm, pale yellow tissue.
Laboratory results:
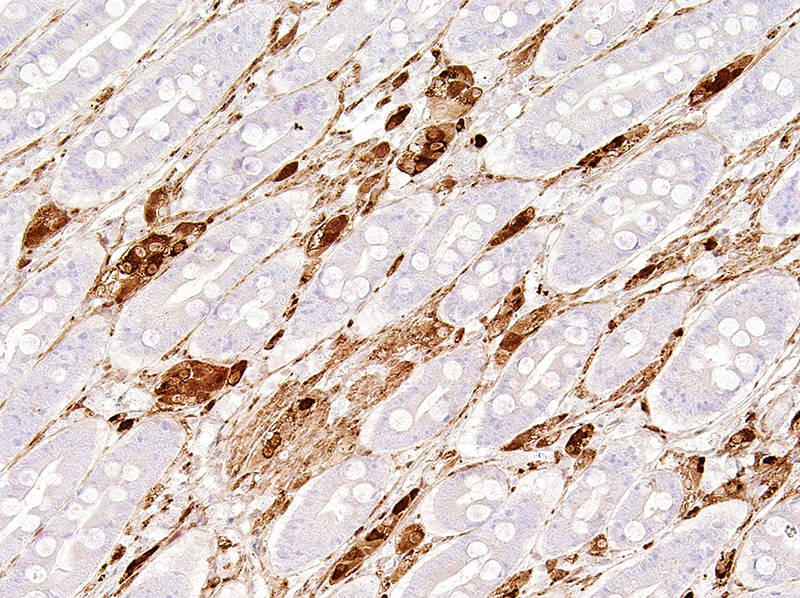
Immunohistochemistry with antibody against NSE confirmed the abnormal presence of neural elements in the small intestinal lamina propria, most notably clusters of neuronal cell bodies but also streaming bundles of axons.
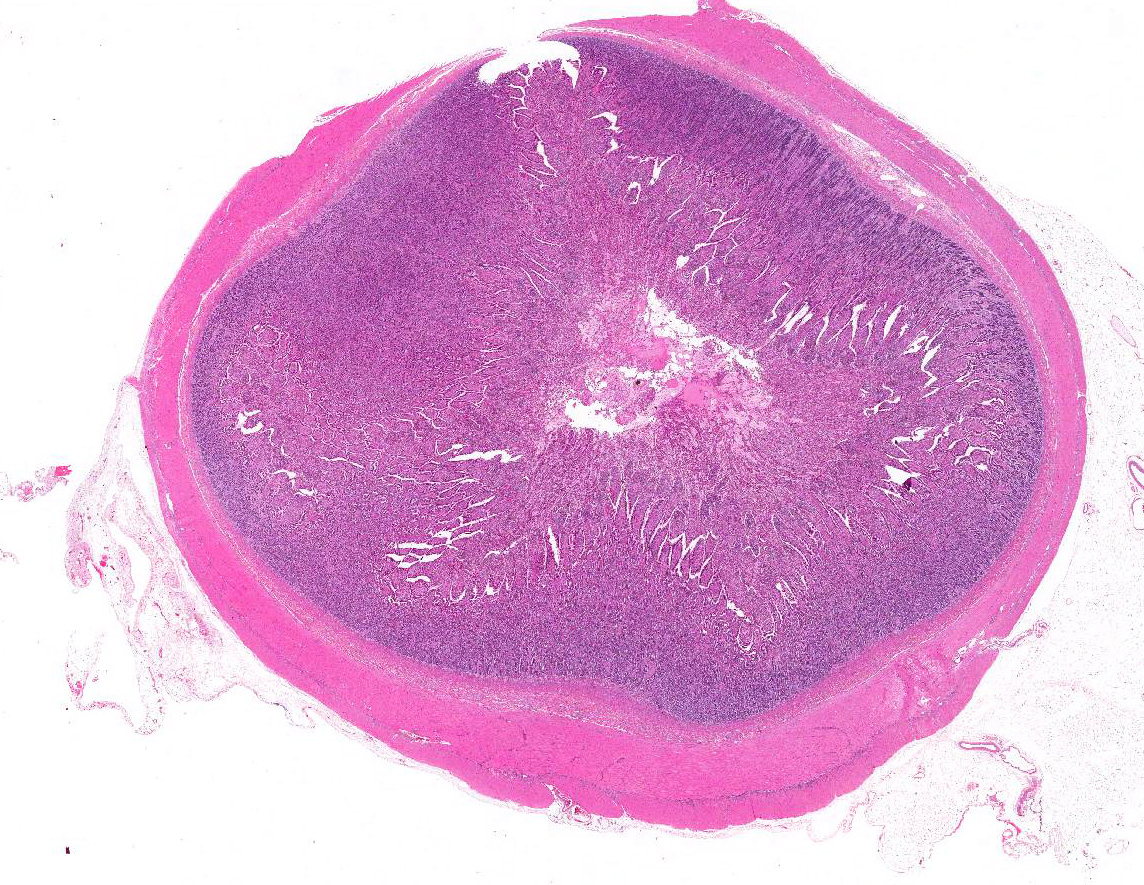
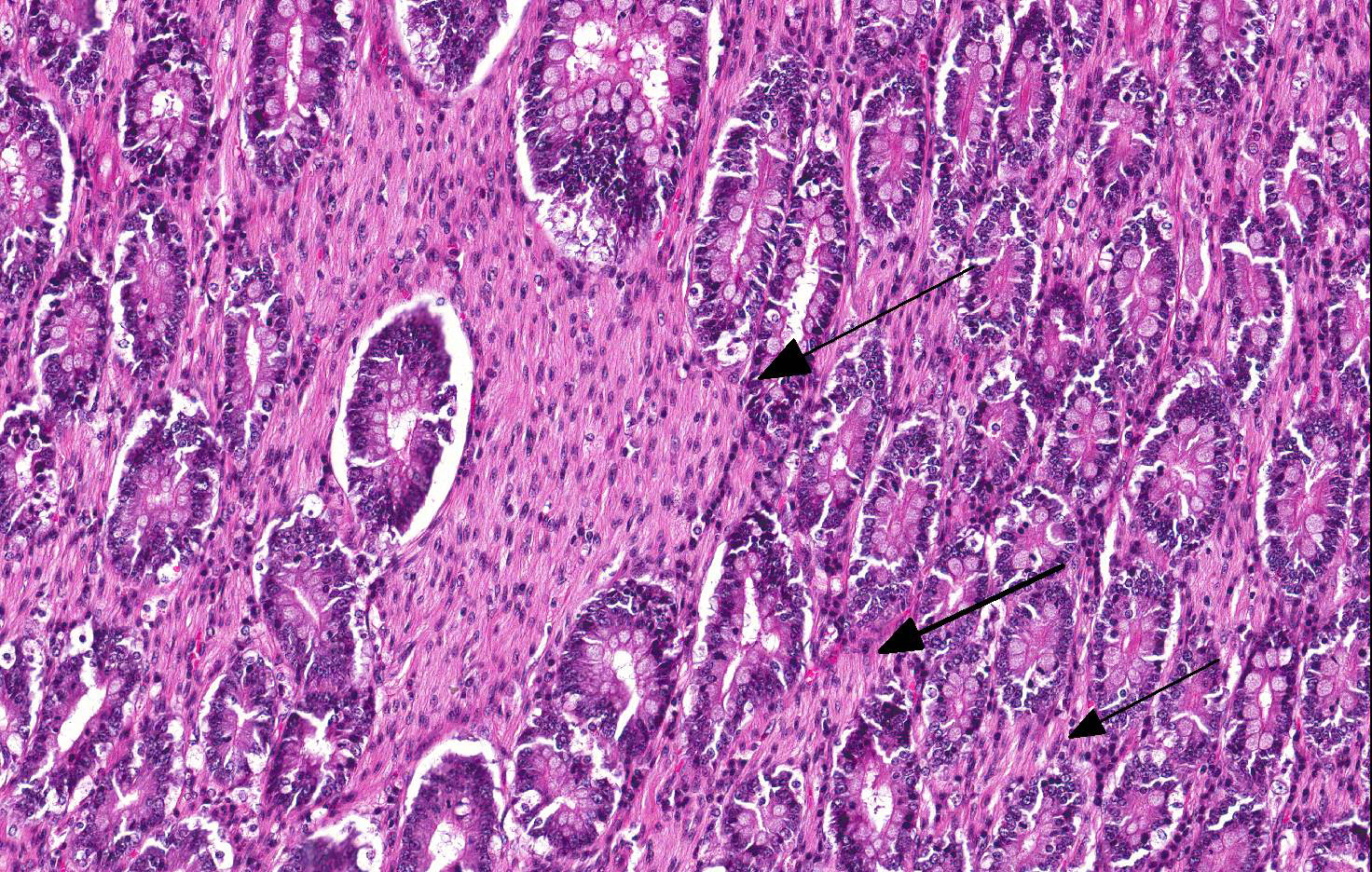
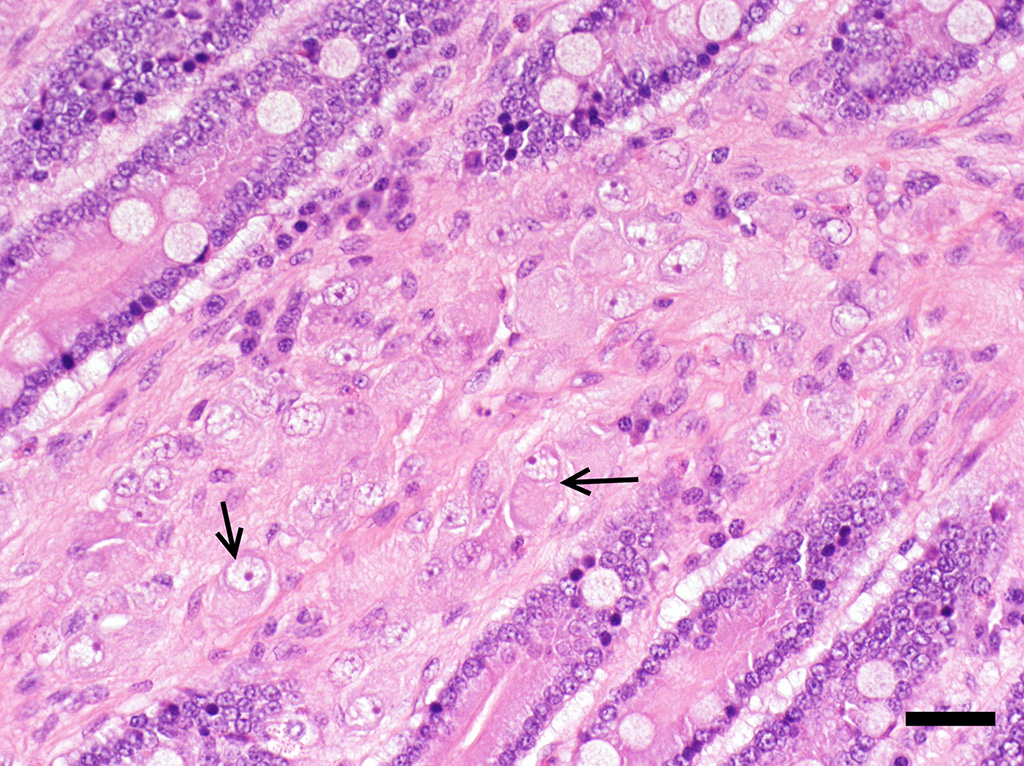
Microscopic Description: Small intestine (jejunum): Numerous well differentiated neuronal cell bodies are scattered throughout the intercryptal lamina propria. The cell bodies are polygonal with faintly visible cell borders, a moderate amount of cytoplasm, large, oval, eccentrically located, hypochromatic, vesiculated to open-faced nuclei and clearly visible nucleoli. There are numerous short streams and nodular aggregates of elongated cells with abundant eosinophilic, fibrillar to foamy cytoplasm, compatible with bundles of axons or Schwann cells. They form nests within the subcryptal area but extend as streams into the mid lamina propria. The myenteric and submucosal plexuses are increased in number and size. There is moderate goblet cell hyperplasia and the lumen contains abundant fibrillar, eosinophilic, mucinous material (mucus). In some sections, rare dilated crypts contain small amounts of necrotic cell debris.
Note: Sections from the most severely thickened portion were a little different (not included here). In addition to the above lesions, there was marked superimposed mucosal hyperplasia, characterized by an increased number of well differentiated but elongated or branching crypts, often lined by numerous goblet cells. Some crypts were distended by necrotic cell debris and/or mucus. Crypt rupture had led to release of lakes of mucus and necrotic cell debris into the lamina propria. There was also marked villous blunting and fusion, with ulceration.
Contributor’s Morphologic Diagnosis:
Jejunum: Intestinal ganglioneuromatosis
Contributor’s Comment: Intestinal ganglioneuromatosis (GN) is a rare condition in which there is hyperplasia of all components of the intestinal ganglia. It has some similarities with ganglioneuroma, which is also the result of neuronal and axonal proliferation but is considered neoplastic.7 However, in contrast to ganglioneuroma, which tends to be well defined and mass-like, GN is poorly demarcated and more diffuse.5 In humans, diffuse GN presents in one of two forms, transmural or mucosal. Transmural lesions also have a more guarded prognosis due to their link with multiple endocrine neoplasia (MEN) IIb and neurofibromatosis 1 (NF1).5,19 GN usually arises in the colon or rectum in humans, though one report described lesions extending from lips to rectum.3,19 Reported clinical signs have included vomiting, weight loss, diarrhea or constipation, hematochezia, melena and abdominal pain.1,4 Similar clinical presentations have been described in veterinary patients.7,8
The few previous reports of canine GN have been in juveniles and have typically only involved the colon.2,7 Small intestinal GN is very rare in the dog, with only one report in the literature.8 In general, alimentary GN in the dog tends to mirror the transmural form in humans, involving the submucosa and intestinal wall, with relative sparing of the mucosa. Our case was a little different in that the lesion arose in a mature dog with no prior history of gastrointestinal disease; it predominantly affected the mucosal lamina propria of the small intestine; and it was successfully treated. In all prior cases it has been speculated that the lesions are probably congenital, based upon the age of the patient and the slowly progressive nature of the disease.2
As mentioned above, MEN IIb, which is the result of a point mutation in the RET proto-oncogene, accounts for the majority of intestinal GN cases in humans. However, the exact pathogenesis of GN is still unclear. Proposed theories include overexpression of neural growth factor leading to proliferation of one nerve fiber type; hyperplasia of multiple nerve fibers, rather than “clonal expansion” of a single subtype; decreased expression of the tumor suppressor gene, PTEN; and increased expression of glial cell line-derived neurotrophic factor (GDNF) and a related neurotrophic factor, neurturin.6,9,12,16 In humans, clinical signs attributable to GN develop within the first weeks of life and are followed by skeletal abnormalities, thickened lips, mucosal neuromas, and a Marfanoid habitus. Multiple endocrine neoplasms occur in later life, including medullary thyroid carcinoma and pheochromocytoma.10 There were no clinical signs to suggest multiple endocrine neoplasia in this case and the adrenal glands were ultrasonographically normal. The etiology of diarrhea associated with neural tumors is still unclear. Increased levels of vasoactive intestinal polypeptide (VIP) have been identified in some cases of human GN, resulting in the clinical syndrome of watery diarrhea, hypokalemia and achlorhydria.11 Alternative mechanisms for the diarrhea include altered intestinal motility, hypersecretion and malabsorption.
JPC Diagnosis: Jejunum: Ganglioneuromatosis.
Conference Comment: Intestinal ganglioneuromas arise in peripheral ganglia and are composed of well-differentiated neurons and nerve processes, Schwann cells, and enteric glial cells. Exceedingly rare in domestic animals, intestinal ganglioneuromatosis (GN) is characterized as regional or segmental proliferation of ganglioneuromatous tissue and has only been reported in dogs, a Boer goat18, a piglet17, and a horse15. Immunohistochemical stains aid in the identification of the individual cell types, with neurons positive for neuron specific enolase (NSE) and S-100 and Schwann and enteric glial cells positive for S-100 and glial fibrillary acidic protein (GFAP).15 These benign proliferative lesions occur predominately in the ileum or colon with few reports of small intestinal infiltration13 and can usually be associated microscopically with the myenteric plexus from which they either extend through the outer portion of the tunica muscularis to the serosal surface15 or through the inner portion of the tunica muscularis, submucosa, and muscularis mucosa, and into the lamina propria13 where it can result in chronic-intestinal pseudoobstruction (CIPO) due to polypoid to segmental expansion of the lamina propria.14 The pathogenesis of GN is currently unknown; however, researchers have identified deletion of PTEN in the enteric nervous system (ENS) of affected mice which corresponds to abnormally low PTEN expression in humans with enteric GN causing CIPO. PTEN is a phosphatase that controls cell growth, proliferation, and death. In these mice, the clinical changes of CIPO were reversed by administration of a pharmacological inhibitor of the PI3K/PTEN-AKT-S6K signaling pathway, indicating a potential therapeutic target for ganglioneuromatous forms of CIPO.14 Additionally, current literature reveals that segmental GN can be removed surgically resulting in resolution of clinical signs and a successful outcome.13
Contributing Institution:
Veterinary Pathology Unit
Division of Veterinary Clinical Sciences
Roslin Institute and Royal (Dick) School of Veterinary Studies
The University of Edinburgh
Easter Bush Veterinary Centre
Roslin
Midlothian
EH25 9RG
UK
http://www.ed.ac.uk/schools-departments/vet/services/pathology
References:
- Atluri DK, Ganesan S, Ferguson RD. Education and imaging. Gastrointestinal: intestinal ganglioneuromatosis. J Gastroenterol Hepatol. 2008;23:160.
- Bemelmans I, Kury S, Albaric O, et al. Colorectal hamartomatous polyposis and ganglioneuromatosis in a dog. Vet Pathol. 2011;48:1012-1015.
- Carney JA, Go VL, Sizemore GW, Hayles AB. Alimentary-tract ganglioneuromatosis — a major component of the syndrome of multiple endocrine neoplasia, type 2b. N Engl J Med. 1976;295:1287-1291.
- Cohen MS, Phay JE, Albinson C, et al. Gastrointestinal manifestations of multiple endocrine neoplasia type 2. Ann Surg. 2002;235:648-654.
- D’Amore ESG, Manivel JC, Pettinato G, Niehans GA, Snover DC: Intestinal ganglioneuromatosis: mucosal and transmural types. A clinicopathologic and immunohistochemical study of six cases. Hum Pathol. 1991;22:276–286.
- DeSchryver-Kecskemeti K, Clouse RE, Goldstein MN, et al. Intestinal ganglioneuromatosis. A manifestation of overproduction of nerve growth factor. N Engl J Med. 1983;308:635-639.
- Fairley RA, McEntee MF. Colorectal ganglioneuromatosis in a young female dog (Lhasa Apso). Vet Pathol. 1990;27:206-207.
- Hazell KLA, Reeves MP, Swift IM. Small intestinal ganglioneuromatosis in a dog. Aust Vet J. 2011;89:15-18.
- Lee NC, Norton JA. Multiple endocrine neoplasia type 2b: Genetic basis and clinical expression. Surg Oncol. 2000;9:111–118.
- Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med. 2011;13:755-764.
- Moon SB, Park KW, Jung SE, et al. Vasoactive intestinal polypeptide-producing ganglioneuromatosis involving the entire colon and rectum. J Pediatr Surg. 2009;44:e19-21.
- O'Donnell AM, Puri P. A role for PTEN in paediatric intestinal dysmotility disorders. Pediatr Surg Int. 2011;27:491-493.
- Paris JK, McCandlish IA, Schwartz T, Simpson JW, Smith SH. Small intestinal ganglioneuromatosis in a dog. J Comp Pathol. 2013;148(4):323-328.
- Piug I, Champeval D, De Santa Barbara P, Jaubert F, Lyonnet S, Larue L. Deletion of Pten in the mouse enteric nervous system induces gangloneuromatosis and mimics intestinal pseudoobstruction. J Clin Invest. 2009;119(12):3586-3596.
- Porter BF, Storts RW, Payne HR, Edwards JF. Colonic ganglioneuromatosis in a horse. Vet Pathol. 2007;44(2):207-210.
- Qiao S, Iwashita T, Ichihara M, et al. Increased expression of glial cell line-derived neurotrophic factor and neurturin in a case of colon adenocarcinoma associated with diffuse ganglioneuromatosis. Clin Neuropathol. 2009;28:105-112.
- Quiroga MA, Lozada MO, Madariaga G, Cappucio JA, et al. Ileal ganglioneuromatosis in a piglet: histopathological and immunohistochemical studies. J Comp Pathol. 2014;151(4):380-383.
- Sheley MF, Higgins RJ, Mete A. Transmural ileal ganglioneuromatosis in a young Boer goat (Capra hircus). J Comp Pathol. 2014;151(2-3):190-194.
- Thway K, Fisher C. Diffuse ganglioneuromatosis in small intestine associated with neurofibromatosis type 1. Ann Diag Pathol. 2009;13:50–54.