Signalment:
Gross Description:
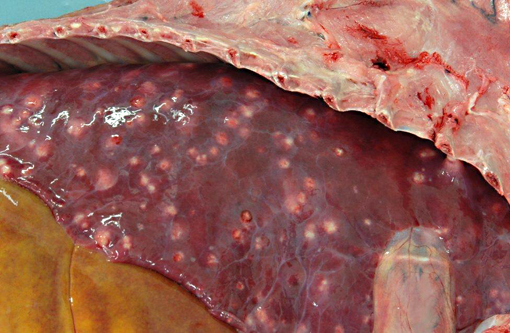
Disseminated throughout the lung lobes were hundreds of 20-50mm diameter, multifocal, firm, off-white to yellow nodules. On the cut section, the nodules were homogenous, off-white and often markedly friable centrally with a thin capsule.Â
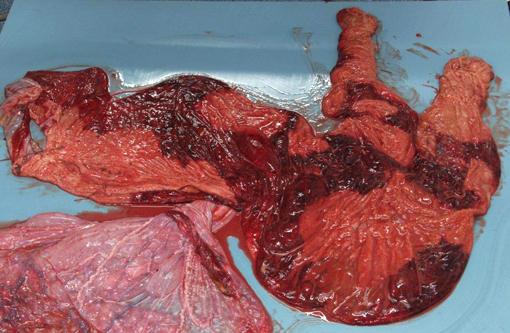
The stroma of the chorioallantois was diffusely variably thickened and expanded by dark red to yellow gelatinous material. The surface of the chorion was mottled with well-demarcated red and tan areas. The chorion was covered by very thin multifocal to coalescing plaques of yellow, friable, foul smelling material. There was abundant, clumped, loosely adhered, tan to yellow material along the amnion, often tracking along blood vessels.Â
Histopathologic Description:
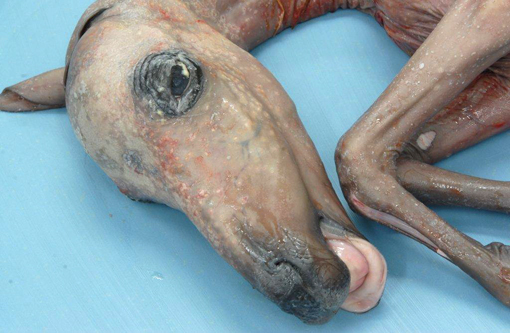
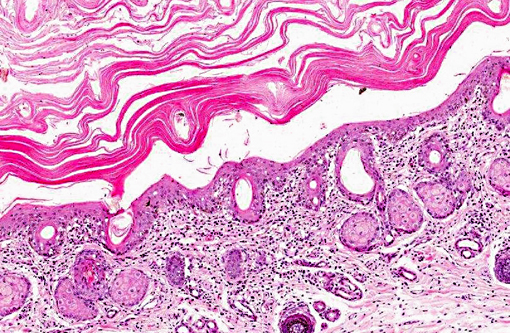
Haired skin: There is marked diffuse orthokeratotic hyperkeratosis. Embedded within the layers of keratin are aggregates of cellular debris and fungal hyphae as in the placenta. Within the superficial dermis, there is a marked, diffuse infiltrate of lymphocytes and plasma cells.Â
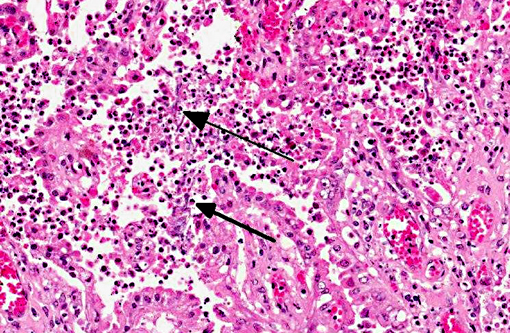
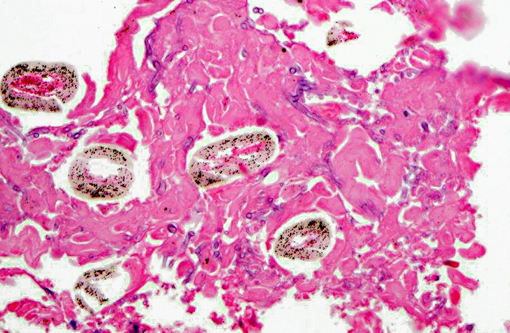
Lung (slide not provided): Randomly scattered throughout the lung are multifocal, nodular areas of inflammation that efface the normal architecture and often engulf adjacent vessels. The centers of the nodules are composed of liquefactive necrosis surrounded by a rim of epithelioid macrophages and multinucleated giant cells admixed with fibrin and hemorrhage. Peripherally, there are moderate numbers of lymphocytes and plasma cells admixed with cellular debris. Entrapped vessels have walls replaced by fibrin and cellular debris. Within the necrotic areas, there are branching clear spaces that are suspicious for fungal hyphae.
Morphologic Diagnosis:
Placenta: Marked, multifocal to coalescing necrotizing placentitis with intralesional fungal hyphae and vasculitis.
Dermatitis: Marked, multifocal dermatitis with orthokeratotic hyperkeratosis and intralesional fungal hyphae.
Lung: Marked, multifocal granulomatous pneumonia.
Condition:
Contributor Comment:
Mycotic placentitis is less common than bacterial placentitis in horses and typically results in a regionally extensive placentitis centered on the cervical star, reflecting the ascending nature of the infection.(2,3) Abortion due to mycotic placentitis occurs more commonly in late gestation and results from placental failure.(3) Mycotic dermatitis is a common concurrent finding. Pulmonary lesions, as seen in this case, are uncommon and are thought to occur as a result of aspiration of contaminated amniotic fluid. Systemic dissemination is rarely seen in the fetus.(4) Aspergillus spp. are the most common fungal organisms isolated from aborted fetuses.(2) In this case, the morphology of fungal hyphae is highly suggestive of Aspergillus spp.; however, cultures are required for definitive diagnosis.Â
JPC Diagnosis:
1. Placenta: Placentitis, necrotizing and suppurative, diffuse, moderate, with focal infarction, vasculitis, fibrinoid change, and moderate numbers of fungal hyphae.
2. Haired skin, eyelid: Dermatitis, lymphoplasmacytic, diffuse, mild, with marked epithelial hyperkeratosis and intracorneal fungal hyphae.
Conference Comment:
There are many noninfectious causes of pregnancy failure in horses, some of which include: twinning, umbilical cord anomalies, endometrial fibrosis, premature placental separation, and fetal thyroid hyperplasia and musculoskeletal disease.(1) Twinning results in a decreased surface area of chorionic villi, since villi do not develop in the areas where the two placentas come into contact. In these cases, death is thought to be due to placental insufficiency. Equine umbilical cord anomalies such as excessive or inadequate length and torsion can also result in failure of pregnancy. Endometrial fibrosis, usually a result of past endometriosis, precludes adequate maternofetal interface, thus resulting in either failure of the mare to become pregnant or an inability to carry the fetus to term. Premature placental separation results in a detachment of the caudal part of the chorioallantois from the uterus and a tearing across the body of the placenta instead of at the cervical star. Fetal anomalies are rare in horses; however, in some regions foals abort due to thyroid hyperplasia and musculoskeletal disease, characterized by microscopic thyroid hyperplasia but no macroscopic gland enlargement. Prognathia, flexural deformities, joint laxity, and tendon ruptures can occur in this syndrome.(1)
Although not as common, there have also been several abortion storms in mares associated with eastern tent caterpillars (ETC), referred to as mare reproductive loss syndrome (MRLS), the pathogenesis for which has not been fully elucidated. Current hypotheses propose either a bacterial invasion and bacteremia occurrence secondary to injury from ingested ETC-related toxins or from mechanical damage to the gastrointestinal mucosa by setae (hairs) on the ETC.(5)
References:
2. Hong CB, Donahue JM, Giles RC Jr, Petrites-Murphy MB, Poonacha KB, Roberts AW, et al. Equine abortion and stillbirth in central Kentucky during 1988 and 1989 foaling seasons. J Vet Diagn Invest. 1993;(4):560-6.
3. Hong CB, Donahue JM, Giles RC Jr, Petrites-Murphy MB, Poonacha KB, Roberts AW, et al. Etiology and pathology of equine placentitis. J Vet Diagn Invest. 1993;(1):56-63.
4. Schlafer DH, Miller RB. Female genital system. In: Maxie MG, ed. Pathology of Domestic Animals. 5th ed. Edinburgh, UK: Saunders Elsevier; 2007:508-509.
5. Sebastian MM, Bernard WV, Riddle TW, Latimer CR, Fitzgerald TD, Harrison LR. REVIEW paper: mare reproductive loss syndrome. Vet Pathol. 2008;45(5):710-22.