Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 15
22 January 2020
Dr.
Cory Brayton, DVM, PhD, DACVP, DACLAM,
Director, Phenotypic Core
Associate Professor of Molecular and Comparative and Pathobiology
Johns Hopkins School of Medicine
733 N. Broadway
Baltimore MD, 21205
CASE I: 16N131-1 (JPC 4128009).
Signalment: 3.5mo old, female, NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD-SCID-gamma/NSG) mouse (Mus musculus)
History: This mouse was xenografted in the mammary fat pad at 6 weeks of age with tumor cells from a breast cancer patient. The mouse presented moribund, hunched and scruffy two months later, and was subsequently euthanized.
Gross Pathology: The xenograft tumor was not observed at gross necropsy. The mouse was in poor body condition, with marked depletion of external and internal adipose stores. The lungs were mottled dark red, and the spleen was dark red to black and smaller than normal. The small intestine contained small amounts of mucous, and few fecal pellets were present in the descending colon.
Laboratory results: N/A.
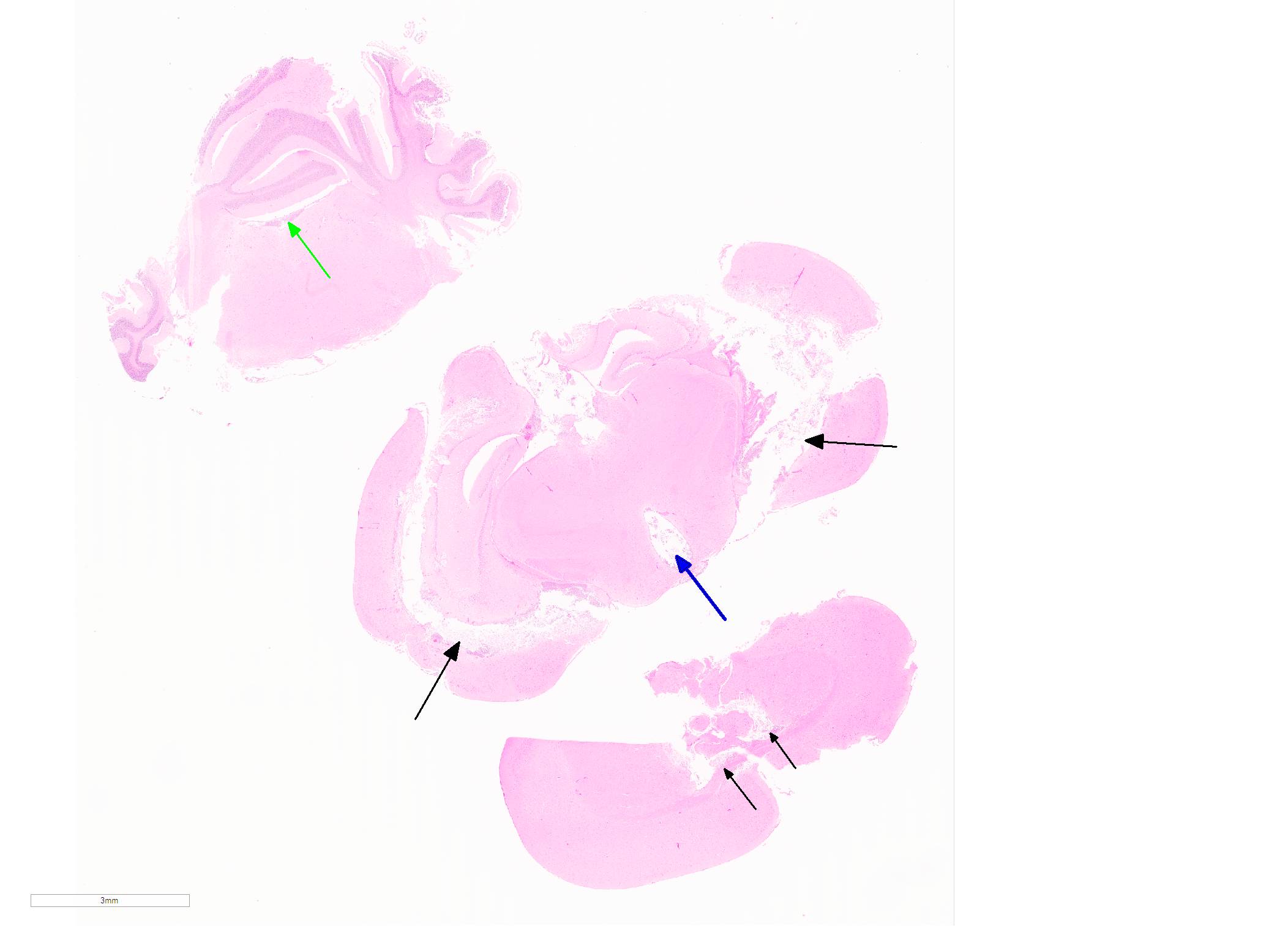
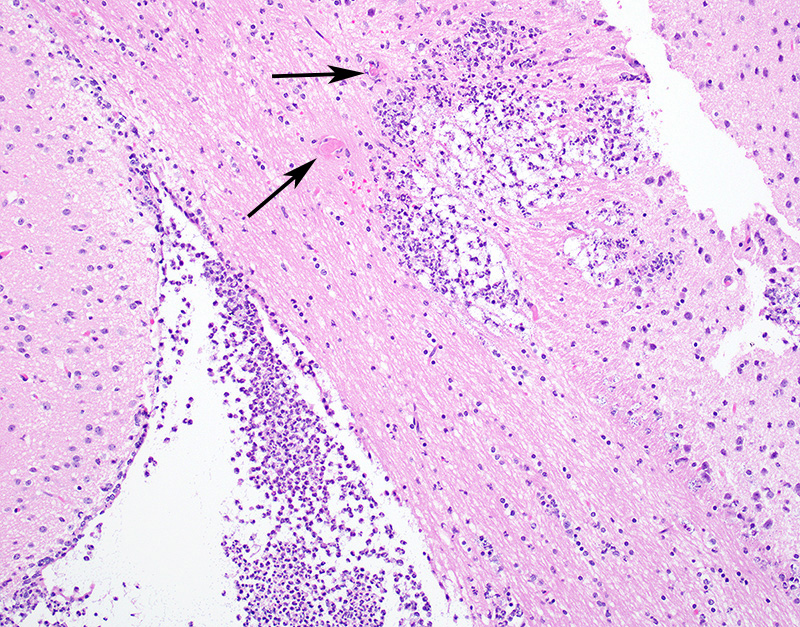
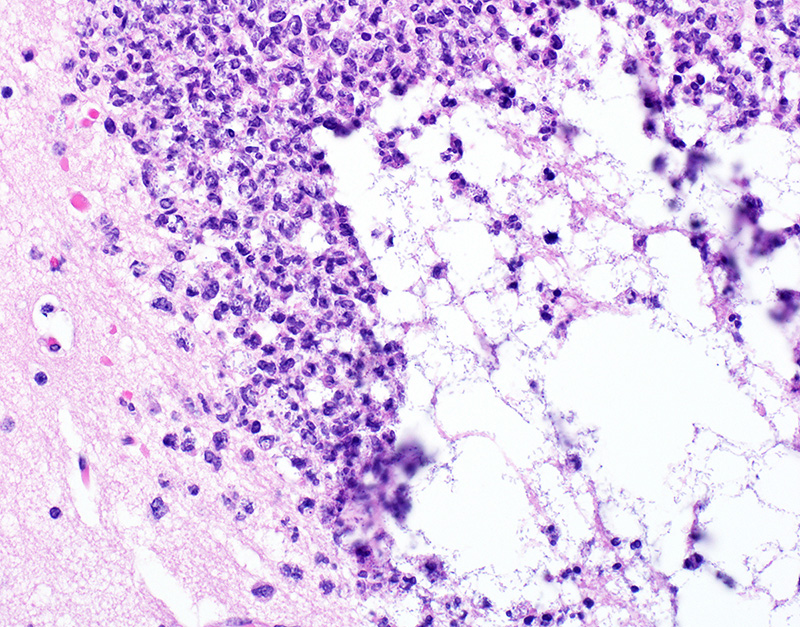
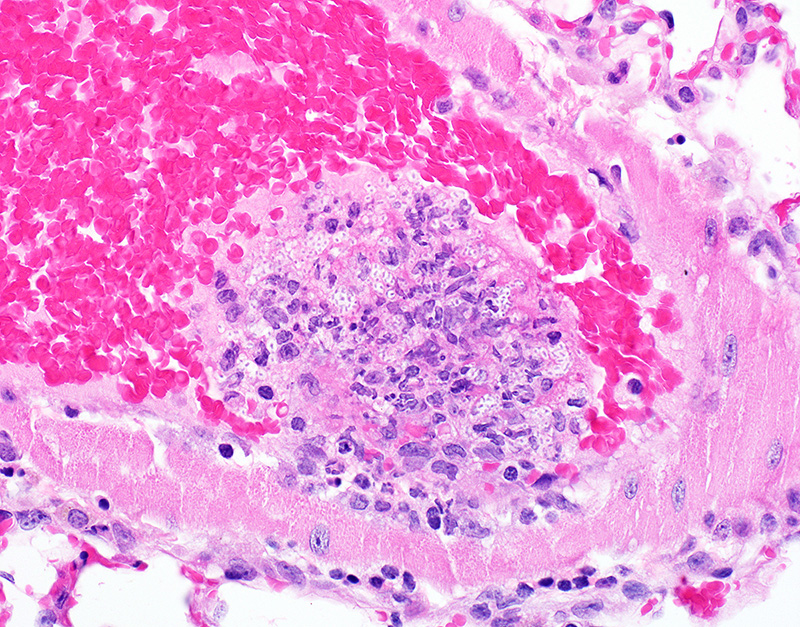
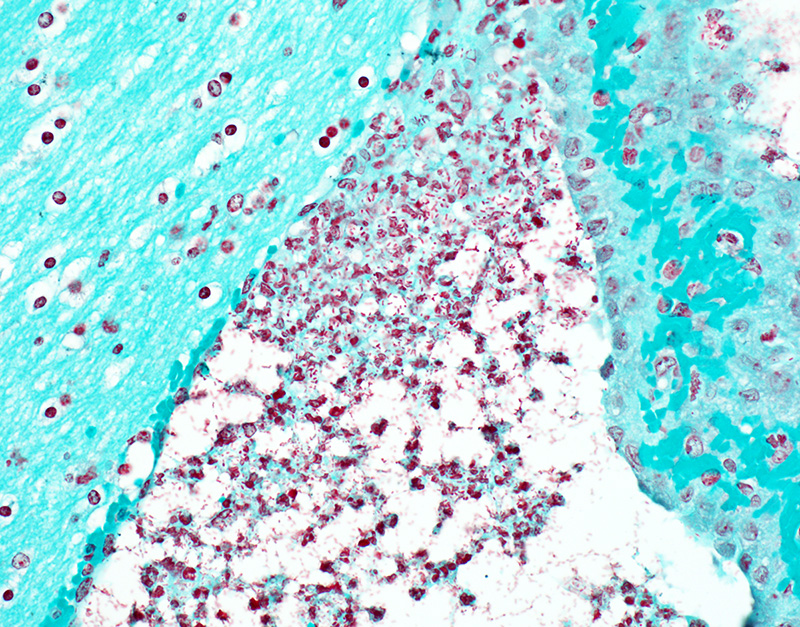
Microscopic Description: In sections of brain, there was a severe inflammatory infiltrate composed exclusively of mature and degenerate neutrophils within the third and lateral ventricles, extending into the subjacent neuropil of the hippocampus and cerebrum with associated fragmentation and rarefaction of the neuropil. Several colonies of short rod-shaped bacteria with peripheral clearing were observed within areas of necrosis. The meninges were expanded with a mild inflammatory infiltrate composed predominantly of neutrophils. In sections of lung, multiple arteries contained variably sized accumulations of fibrin, neutrophils, and fewer macrophages, some containing similar rod-shaped bacteria. The perivascular interstitium and alveolar walls were multifocally thickened with few neutrophils and macrophages, some of which contained intracytoplasmic bacteria. In sections of liver, there were few small inflammatory foci composed of degenerate neutrophils associated with hepatocellular necrosis and rod-shaped bacteria, and there were increased numbers of inflammatory cell infiltrates within portal areas, composed primarily of lymphocytes, macrophages, and fewer neutrophils. Occasionally rod-shaped bacteria were observed in Kupffer cells. In sections of bone marrow, there was diffuse and marked myeloid hyperplasia. Hucker-Twort gram stain on sections of brain revealed gram negative rod-shaped bacteria (Fig4).
Contributor Morphologic Diagnosis:
1. Brain: Meningoencephalitis, suppurative, locally extensive, severe, with rod-shaped bacterial colonies.
2. Lung: Pneumonia, suppurative and embolic, multifocal, moderate, with rod-shaped bacterial colonies.
3. Liver: Hepatitis, suppurative and embolic, multifocal, minimal, with rod-shaped bacterial colonies.
4. Bone marrow: Myeloid hyperplasia, diffuse, marked.
Contributor Comment: Klebsiella spp. are gram-negative rod-shaped bacteria that are typically commensals in mice, and are not a significant cause of naturally occurring disease. K. oxytoca is ubiquitous in the environment, and can be isolated from the gastrointestinal tract, nasopharynx, lung, skin, and mucous membranes of healthy animals and humans.2,7,14 However, these organisms may become opportunistic pathogens in certain situations, and in both humans and animals infections with K. pneumoniae and oxytoca are most commonly associated with clinical disease. Klebsiella oxytoca can cause suppurative lesions in various organ systems in mice, particularly the reproductive tract, where it has been associated with suppurative endometritis, salpingitis, and perioophortitis often progressing to peritonitis and abscess formation. Other reported opportunistic infections in rats and mice include perianal dermatitis, otitis media, cystitis, pyelonephritis, keratoconjunctivitis, Harderian gland adenitis, oral infection, subcutaneous, abdominal and hepatic abscesses, pneumonia, meningitis, endotoxemia and septicemia. 1,3,8,14 In humans, Klebsiella oxytoca and pneumoniae are considered important causes of nosocomial infections in hospitalized patients, and has been implicated in community acquired pneumonia, adult and neonatal sepsis and bacteremia, septic arthritis, soft tissue abscesses, and urinary tract infections, and chronic nasal infections.1,2,10 K. oxytoca is also suspected to be the etiologic agent responsible for antibiotic-associated hemorrhagic colitis (AAHC) in humans, which occurs following antibiotic therapy and is characterized by bloody diarrhea, abdominal cramping and segmental hemorrhagic typhlocolitis 1,2,14. Antibiotic therapy has been shown to promote abnormal colonization by Klebsiella spp. in humans1 and rodents,5 and an animal model of AAHC has been developed using oral administration of antibiotics in rats followed by oral infection with K. oxytoca.6
Immune status of the host plays an integral role in the pathogenesis of disease by Klebsiella spp. Certain strains and genetically modified mouse lines are more prone to developing lesions associated with Klebsiella spp. infection. Suppurative otitis media, urogenital tract infections, and pneumonia have been reported in substrains of C3H/HeJ mice and NMRI- Foxn1nu mice, and LWE.1AR1 rats, and chronic renal inflammatory lesions and ascending urinary tract infections in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. 1,2,4,7,14 C3H/HeJ substrains are hyporesponsive to lipopolysaccharide (LPS) produced by gram-negative bacteria, due to a single amino acid substitution in the toll-like receptor 4 (TLR4) protein, NMRI- Foxn1nu (nude) mice lack a thymus and are therefore T-cell deficient, and LEW.1AR-iddm rats are at risk for infection secondary to development of diabetes mellitus. Finally, NSG mice have multiple mutations including the Prkdc severe combined immune deficiency (scid) mutation, IL2 gamma deficiency, mutation of the C5 complement gene, and a novel MHC haplotype which leads to lack of normal T and B lymphocytes and NK cells, and deficient cytokine and complement signaling and function.4 Increasing age may also play a contributory role in pathogenesis of Klebsiella infection, as suppurative reproductive lesions due to K. oxytoca infection have been reported in National Toxicology Program (NTP) chronic chemical carcinogenesis bioassays.3
Klebsiella exerts its pathogenic effects by taking advantage of an immunosuppressed or immunocompromised host, and through the use of several virulence factors. The presence of a distinct polysaccharide capsule observed as a peripheral clearing on routine light microscopy is correlated with virulence of the organism, enabling it to resist phagocytosis and bactericidal components of serum.1,7,10 In addition, several in vitro studies in humans and in mice have shown that K. oxytoca produces a cytotoxin, tilivalline, which induces cell death through inhibition of DNA synthesis.2,14 Furthermore, genomic studies on tilivalline-producing K. oxytoca in both humans and mice revealed a number of genes associated with virulence potential.2 Finally, a substrain of K. oxytoca (TNM3) has been shown to produce an immunosuppressive polysaccharide, AZ9, which is associated with decreased IL4 and IFNg responses leading to an overall depressed Th2-type immune response.11,12
In the present case, lesions were clearly representative of a septicemic process with multiple suppurative and embolic lesions in several organs including the lung, liver, and brain, and a myeloid response in the bone marrow. By light microscopy and gram staining, the observed peripheral clearing around the gram-negative, rod-shaped bacterial organisms is characteristic of Klebsiella spp. The affected animal in this case was a NSG mouse, which as previously discussed is a severely immunodeficient mouse model prone to opportunistic infection. These mice are commonly used to model development, progression, and metastasis of human tumor cells in vivo, through xenotransplantation experiments. The source of the infection is unknown, but it is possible that K. oxytoca was introduced through human contact as this organism can be spread from humans to rodents,1 or through environmental contamination. One major concern with the presence of such opportunistic organisms in animals maintained with a well-defined hygienic status (specific pathogen-free) is that they are becoming more common and relevant as causes of disease in such colonies,1 so knowledge of these organisms is imperative to maintaining high standards of animal welfare and research quality. Furthermore, in experiments utilizing immunocompromised mice for serial tumor passage or xenograftment, transfer of tumor cells or other biologic material contaminated with infectious agents such as Klebsiella is a potential significant risk factor to the health recipient mice, as well as the quality of the experimental outcome due to morbidity and mortality secondary to opportunistic, and unexpected, infection.
Contributing Institution:
In Vivo Animal Core, Unit for Laboratory Animal Medicine
University of Michigan Medical School
Ann Arbor, MI 48109
http://animalcare.umich.edu/business-services/vivo-animal-core
JPC Diagnosis: Cerebrum: Ventriculitis, periventriculitis, and meningitis, necrotizing and suppurative, multifocal to coalescing, severe, with thrombosis, gliosis, and numerous bacilli.
JPC Comment: The contributor has provided an outstanding and thorough review of this opportunistic infection in laboratory rodents, especially immunosuppressed models.
Tilvallin, as mentioned bythe contributor, is a gene product of K. oxytoca which induces apoptosis and loss of barrier integrity in vitro in human epithelial cells, which suggests a possible pathogenesis inhuman antibiotic-associated hemorrhagic colitis (AAHC).12 Further investigation of the biosynthesis of this compound has demonstrated a number of other secondary metabolites, also pyrrolobenzodiazpines (PBD), including tilmysin and culdesacin. The combination of tilmysin with indole actually yields tilvallin. The PBD family of compounds are potent cytotoxic agents which demonstrate both antibacterial and anticancer activity due to DNA alkylation and formation of PBD-DNA adducts. In addition, tilvallin is also a microtubule stabilizing agent, a class of compunds that shift the balance of cellular tubulin from soluble to polymerized and are widely used as anticancer agents. This class also includes taxol, a widely used chemotherapeutic12.
In 2011, the Enterobacteriaceae genus Raoultella (named after the French bacteriologist Didier Raoult) was separated from Klebsiella through the use of molecular techniques, as well as the identification of growth at 10o C and use of L-sorbose as a carbon source). This genus include four species: Raoultella ornithinolytica, R. planticola, R. terrigena, and R. electrica.9 These bacilli are found in plants and soli in aquatic environments. R. ornithinolytica and R. planticola are considered emerging human pathogen, which results in biliary tract infections in elderly or immunosuppressed patients with malignancies or who have undergone invasive procedures. It is considered likely that a number of Klebsiella infections diagnosed historically may actually be of bacilli of this genus.9
The moderator discussed Rodentibacter pneumotropica as a common opportunist in immunosuppressed mice (which is usually difficult to see on HE and special stains), which highlights that the name of this common opportunist has changed within the last few years (for those who are trying to keep up with the microbiologists. The moderator also commented on the description of a ?smaller than normal? spleen, as the normal weight of the spleen of an immunocompent mouse is 0.2g, and of a NOD-SCID mouse is 0.02g, highlighting not only the size difference of spleens, but the need to use absolute weights in this species. As this was a xenograft animal, the moderator wondered about the possibility of irradiation prior to engraftment in this individual further complicating this picture. Unfortunately lab data was not available in this case to distinguish between K. oxtyoca and K. pneumoniae in this case, so the moderator was loathe to pick one species over the other.
References
1 Bleich A, Kirsch P, Sahly H, Fahey J, Smoczek A, Hedrich HJ, et al.: Klebsiella oxytoca: opportunistic infections in laboratory rodents. Lab Anim 2008:42(3):369-375.
2 Darby A, Lertpiriyapong K, Sarkar U, Seneviratne U, Park DS, Gamazon ER, et al.: Cytotoxic and pathogenic properties of Klebsiella oxytoca isolated from laboratory animals. PLoS One 2014:9(7):e100542.
3 Davis JK, Gaertner DJ, Cox NR, Lindsey JR, Cassell GH, Davidson MK, et al.: The role of Klebsiella oxytoca in utero-ovarian infection of B6C3F1 mice. Lab Anim Sci 1987:37(2):159-166.
4 Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD: Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol 2011:48(2):495-499.
5 Hansen AK: Antibiotic treatment of nude rats and its impact on the aerobic bacterial flora. Lab Anim 1995:29(1):37-44.
6 Hogenauer C, Langner C, Beubler E, Lippe IT, Schicho R, Gorkiewicz G, et al.: Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 2006:355(23):2418-2426.
7 MacArthur CJ, Pillers DA, Pang J, Degagne JM, Kempton JB, Trune DR: Gram-negative pathogen Klebsiella oxytoca is associated with spontaneous chronic otitis media in Toll-like receptor 4-deficient C3H/HeJ mice. Acta Otolaryngol 2008:128(2):132-138.
8 Percy DH, Barthold S. W.: Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IA: Blackwell Publishing; 2016: 50-72.
9. Ponce-Alonse M. Rodrigues-Rojas L, del Campo R, Canton, R, Morosini MI. Comparison of different methods for identificatio of species of the genus Raoultella: report of 11 cases of Rasoultella causing bacteraemia and literature review. Clin Microbiol Infect 2016; 22:252-257.
10 Sahly H, Podschun R: Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin Diagn Lab Immunol 1997:4(4):393-399.
11 Sugihara R, Matsumoto Y, Ohmori H: Suppression of IgE antibody response in mice by a polysaccharide, AZ9, produced by Klebsiella oxytoca strain TNM3. Immunopharmacol Immunotoxicol 2002:24(2):245-254.
12 Sugihara R, Oiso Y, Matsumoto Y, Ohmori H: Production of an immunosuppressive polysaccharide, AZ9, in the culture of Klebsiella oxytoca strain TNM3. J Biosci Bioeng 2001:92(5):485-487.
13. Unterhauser K, Potil L, Scheiditz GS, KKeienesberger S. et al. Klebsiella oxytoca enterotoxins tilimycin and tilivalline have distinct host DNA-damaging and microtubule-stabilizing activities. Proc Natl Acad Sci USA 2019; 116(9): 3774-3783.
14 Whary M, Baumgarth, N., Fox, J. G., Barthold, S. W.: Biology and Diseases of Mice. In: Fox JF, Anterson, L. C., Otto, G. M., Pritchett-Corning, K. R., Whary, M. T., ed. Laboratory Animal Medicine. 3rd ed. Amsterdam: Academic Press; 2015.