Signalment:
Gross Description:
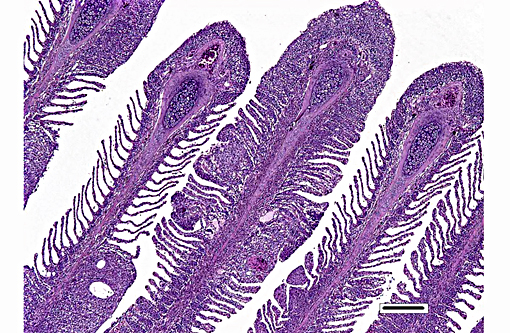
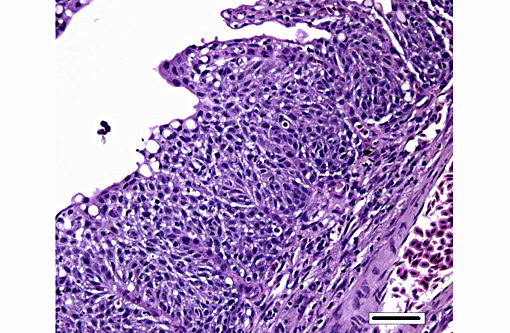
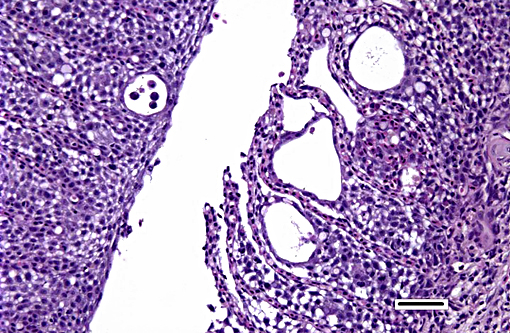
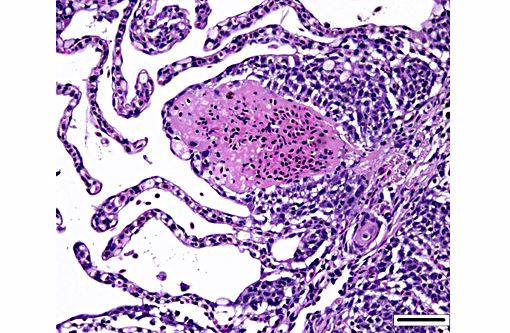
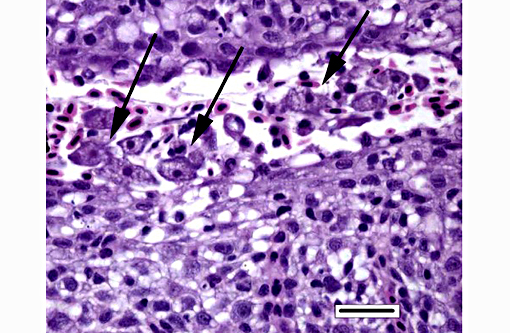
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
AGD affects salmonids, turbot (Scophthalmus maximus), ayu (Plecoglossus altivelis), European seabass (Dicentrarchus labrax), sharpsnout seabream (Diplodus puntazzo), and ballan wrasse (Labrus bergylta).(12,21) In this review, we will focus on Atlantic salmon (Salmo salar), as this is the species in which this disease has the highest economic impact. In S. salar, the downstream effects of AGD include reduced growth, increased susceptibility to other pathogens and, if untreated, mortality. Its economic burden is large; in Tasmania (where AGD has been endemic since 1980) costs have been estimated at ~$1 AUS/kg salmon (not cited). Disease occurs at temperatures 12-20oC and high salinity (35o/oo).(17) However, there is variability on this presentation and in Scotland outbreaks may happen as low as 8oC.
Grossly, AGD presents as multifocal white to grey swollen areas on the gills, associated with excess of mucus.(17) Lesion distribution favours gill areas with lower water flow (i.e. at the dorsal aspect of the gill arches).(3) Several gross scoring systems have been developed, with diverse focus on aspects of the severity and distribution of AGD-associated lesions.(2,30) These scoring systems have been used to predict expected mortality levels of AGD affected fish if left untreated(30). There is moderate to good agreement (κ=0.52-0.74) between the gross signs of AGD and the histological presentation, and regions with gross lesions are those with histological signs.(1) However, this agreement is not ideal, with multiple instances of disagreement depending on the AGD stage, other pathogens, assessor, sampling method, histological technique and histological experience.(1)
Histologically, AGD presents with lamellar epithelial hyperplasia and fusion (with creation of small interlamellar vesicles/lacunae), which are variably associated with interbranchial lymphoid tissue area increase, filament fusion, changes in chloride cells, and hemorrhage.(4,17,22). Gill epithelial hyperplasia is a stereotypical feature of gill damage, but in AGD it has been postulated that it may be a result of the effect of the cytotoxic protease of the amoeba.(7) Whether this is true or note, gill lamellar hyperplasia in AGD is associated with proliferating cell nuclear antigen (PCNA) increase(2), and p53 downregulation(18) in lamellar epithelial cells. Amoebic organisms are noted in close association with the epithelium of lamellae and filaments, and often around the margins of the hyperplastic plaques.(4) There is no description of epithelial invasion and these amoeba are considered ectoparasites.(16) In field studies, Adams et al.(2) first detected histological lesions at 13 week post transfer, with three distinct phases: (1) Primary attachment/interaction associated with extremely localized host cellular alterations, juxtaposed to amoebae, including epithelial desquamation and oedema; (2) Initial focal hyperplasia of undifferentiated epithelial cells; and (3) lesion expansion, squamationstratification of epithelia at lesion surfaces and variable recruitment of mucous cells to these regions. A pattern of preferential colonization of amoebae at lesion margins was apparent during stage 3 of disease development.
There is a significant body of work on the systemic host response during AGD. Early work on innate immunity changes in S. salar recorded an increased chemotactic response of head kidney macrophages, with paradoxical reduction in head kidney phagocyte respiratory burst.(11) Overall, there is debate on the protective role of adaptive immunity in AGD.(26) There is evidence of anti- N.perurans antibodies circulating post exposure, but the response is considered poor.(10,31) A field study on the adaptive immune response to AGD revealed that even though there is increase of seropositivity against N. perurans (with predominance of carbohydrate epitopes over peptide epitopes), there was no evidence to support that these antibodies were protective.(29) Subsequently, Vincent et al.(31) noted reduced mortality in the second exposure which may support the existence of a protective acquired immune response. Interestingly this reduction in mortality was not associated with reduction in the severity of the gill lesions.
At the gill level, there is increased mRNA expression of IL-1β, TCR-alpha chain, CD8, CD4, MHC-IIα, MHC-I, IgM and IgT in AGD-challenged salmon at 10 days post-inoculation.(23) This suggests that an acquired immune response is present at the local levewhich is further supported by the presence of MHC class II+ cells within gill lesions.(19) An interesting pattern has been noted with the role of IL-1β in AGD, which is overexpressed in advanced AGD, despite an absence of marked inflammation.(6) This may be due to reduced transcription of the receptor IL-1R-I, although this effect is not complete, as IL1R-II is expressed at normal levels.(20)
The pathogenic effect of AGD on S. salar has also been studied, and includes cardiovascular compromise, osmoregulatory changes, and ion regulation changes. AGD results in cardiovascular compromise, which is noted even with light AGD lesions.(13,15) This may explain why mortalities can occur with light lesions, as it happens in the field, and could be related to individual variation in the ability to cope with acute and chronic increases in cardiovascular demand. In AGD, cardiovascular compromise may be the result of increased afterload, characterized by elevation of dorsal aortic pressure and systemic circulation resistance (systemic hypertension), without derangement of the renin-angiotensin system.(13,15,25) The increased afterload is coupled with cardiac morphological changes in chronic AGD. In a field study using gross lesion scores and number of freshwater baths required to assess clinical severity, S. salar with severe AGD history had increased ventricle length: axis length and ventricular width:axis length/height and thickened compact myocardium at the expense of the spongiosa, when compared with fish with light AGD history.(27)
Very interestingly, the cumulative findings of several studies do not support respiratory failure as the main mechanism of AGD-induced mortality. This may be due to gill reserve capacity and reduction in mucus density, despite an increased amount of mucus.(26,28) AGD results in hyperplasia of the lamellar epithelium, with consequent reduction on respiratory-efficient gill surface area due to multifocal increase in blood water diffusion distance.(2) However, this is coupled with reduction on mucus density, which reduces the blood-water diffusion barrier resistance.(28) During AGD, there is increase in the rate and amplitude of opercular movements,(9) but this is not coupled with reduction in oxygen uptake.(26) Other respiratory changes are noted, however, and CO2 plasma pressure is increased in AGD-affected fish, with reduced pH consistent with mild respiratory acidosis from 7dpi.(24), preceded by early alkalosis (48hpi), which could be the result of hyperventilation.(14)
JPC Diagnosis:
Conference Comment:
The moderator also discussed the differences between lamellar fusion and lamellar adhesion. In cases of lamellar fusion, there is filling of lamellar sulci from the bottom and it is most often associated with inflammation and epithelial proliferation. With lamellar adhesions, there is adherence of two or more lamella and there may be an absence of proliferation or inflammation. In this case, there are examples of both with lamellar fusion near the base of lamella and adhesions toward the top.
Dilated capillaries within secondary lamellae were described as either telangiectasia or aneurysmal dilation by conference participants. The moderator indicated this vascular change can be a perimortem event, or a non-specific change in certain diseases. In many cases, distinguishing between peri- and antemortem telangiectasis is difficult. However, in this case, the presence of organizing fibrin thrombi indicates the telangiectacic changes are antemortem.
References:
1. Adams MB, Ellard K, Nowak BF. Gross pathology and its relationship with histopathology of amoebic gill disease (AGD) in farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 2004; 27:151-161.
2. Adams MB, Nowak BF. Amoebic gill disease: sequential pathology in cultured Atlantic salmon, Salmo salar L. J. Fish Dis.2003; 26: 601-614.
3. Adams MB, Nowak BF. Distribution and structure of lesions in the gills of Atlantic salmon, Salmo salar L., affected with amoebic gill disease. J. Fish Dis.2001; 24:535-542.
4. Adams MB, Nowak BF. Sequential pathology after initial freshwater bath treatment for amoebic gill disease in cultured Atlantic salmon, Salmo salar L. J. Fish Dis.2004; 27:163-173.
5. Bridle AR, Davenport DL, Crosbie PB, Polinski M, Nowak BF. Neoparamoeba perurans loses virulence during clonal culture. Int J Parasitol.2015; 45(9-10):575-8.
6. Bridle AR, Morrison RN, Cupit Cunningham PM, Nowak BF. Quantitation of immune response gene expression and cellular localisation of interleukin-1beta mRNA in Atlantic salmon, Salmo salar L., affected by amoebic gill disease (AGD). Vet Immunol Immunopathol.2006; 114: 121-134.
7. Butler R, Nowak BF. In vitro interactions between Neoparamoeba sp. and Atlantic salmon epithelial cells. Journal of Fish Diseases. 2004; 27: 343-349.
8. Crosbie PB, Bridle AR, Cadoret K, Nowak BF. In vitro cultured Neoparamoeba perurans causes amoebic gill disease in Atlantic salmon and fulfils Koch's postulates. Int J Parasitol.2012; 42:511-515.
9. Fisk DM, Powell MD, Nowak BF. The effect of Amoebic Gill Disease and hypoxia on survival and metabolic rate of Atlantic salmon (Salmo salar). Bull Eur Assoc Fish Pathol. 2002; 22: 190-194.
10. Gross K, Carson J, Nowak B. Presence of anti-Neoparamoeba sp. antibodies in Tasmanian cultured Atlantic salmon, Salmo salar L. J. Fish Dis. 2004; 27: 81-88.
11. Gross KA, Powell MD, Butler R, Morrison RN, Nowak BF. Changes in the innate immune response of Atlantic salmon, Salmo salar L., exposed to experimental infection with Neoparamoeba sp. J. Fish Dis. 2005; 28: 293-299.
12. Karlsbakk E, Olsen AB, Einen A-CB, Mo TA, Fiksdal IU, Aase H, Kalgraff C, Sk+â-Ñr S-+â-à, Hansen H: Amoebic gill disease due to Paramoeba perurans in ballan wrasse (Labrus bergylta). Aquaculture. 2013; 412413: 41-44.
13. Leef MJ, Harris JO, Hill J, Powell MD. Cardiovascular responses of three salmonid species affected with amoebic gill disease (AGD). J Comp Physiol B. 2005; 175: 523-532.
14. Leef MJ, Harris JO, Powell MD. Respiratory pathogenesis of amoebic gill disease (AGD) in experimentally infected Atlantic salmon Salmo salar. Diseases of Aquatic Organisms. 2005; 66: 205-213.
15. Leef MJ, Hill JV, Harris JO, Powell MD. Increased systemic vascular resistance in Atlantic salmon, Salmo salar L., affected with amoebic gill disease. J. Fish Dis. 2007; 30: 601-613.
16. Lovy J, Becker JA, Speare DJ, Wadowska DW, Wright GM, Powell MD. Ultrastructural Examination of the Host Cellular Response in the Gills of Atlantic Salmon, Salmo salar, with Amoebic Gill Disease. Vet Pathol. 2007; 44: 663-671.
17. Mitchell SO, Rodger HD. A review of infectious gill disease in marine salmonid fish. J. Fish Dis. 2011; 34: 411-432.
18. Morrison RN, Cooper GA, Koop BF, Rise ML, et al. Transcriptome profiling the gills of amoebic gill disease (AGD)-affected Atlantic salmon (Salmo salar L): a role for tumor suppressor p53 in AGD pathogenesis? Physiol Genomics. 2006; 26: 15-34.
19. Morrison RN, Koppang EO, Hordvik I, Nowak BF. MHC class II+ cells in the gills of Atlantic salmon (Salmo salar L.) affected by amoebic gill disease. Vet Immunol Immunopathol. 2006; 109: 297-303.
20. Morrison RN, Young ND, Nowak BF. Description of an Atlantic salmon (Salmo salar L.) type II interleukin-1 receptor cDNA and analysis of interleukin-1 receptor expression in amoebic gill disease affected fish. Fish Shellfish Immunol. 2012; 32:1185-1190.
21. Munday BL, Zilberg D, Findlay V. Gill disease of marine fish caused by infection with Neoparamoeba pemaquidensis. J. Fish Dis. 2001; 24: 497-507.
22. Norte dos Santos CC, Adams MB, Leef MJ, Nowak BF. Changes in the interbranchial lymphoid tissue of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol.2014; 41: 600-607.
23. Pennacchi Y, Leef MJ, Crosbie PB, Nowak BF, Bridle AR. Evidence of immune and inflammatory processes in the gills of AGD-affected Atlantic salmon, Salmo salar L. Fish Shellfish Immunol. 2014; 36: 563-570.
24. Powell MD, Fisk D, Nowak BF. Effects of graded hypoxia on Atlantic salmon infected with amoebic gill disease. J Fish Biol. 2000; 57: 1047-1057.
25. Powell MD, Forster ME, Nowak BF. Apparent vascular hypertension associated with Amoebic Gill Disease affected Atlantic salmon (Salmo salar) in Tasmania. Bull Eur Assoc Fish Pathol. 2002; 22: 328-333.
26. Powell MD, Leef MJ, Roberts SD, Jonesk MA. Neoparamoebic gill infections: host response and physiology in salmonids. J Fish Biol. 2008; 73: 2161-2183.
27. Powell MD, Nowak BF, Adams MB. Cardiac morphology in relation to amoebic gill disease history in Atlantic salmon, Salmo salar L. J. Fish Dis. 2002; 25: 209-215.
28. Roberts SD, Powell MD. The viscosity and glycoprotein biochemistry of salmonid mucus varies with species, salinity and the presence of amoebic gill disease (vol 175, pg 1, 2004). Erratum in: J Comp Physiol B. 2005; 175: 219-219.
29. Taylor RS, Crosbie PB, Cook MT. Amoebic gill disease resistance is not related to the systemic antibody response of Atlantic salmon, Salmo salar L. J. Fish Dis. 2010; 33: 1-14.
30. Taylor RS, Muller WJ, Cook MT, Kube PD, Elliott NG. Gill observations in Atlantic salmon (Salmo salar, L.) during repeated amoebic gill disease (AGD) field exposure and survival challenge. Aquaculture. 2009; 290: 1-8.
31. Vincent BN, Morrison RN, Nowak BF. Amoebic gill disease (AGD)-affected Atlantic salmon, Salmo salar L., are resistant to subsequent AGD challenge. J. Fish Dis. 2006; 29:549-559.
32. Young ND, Crosbie PBB, Adams MB, Nowak BF, Morrison RN. Neoparamoeba perurans n. sp., an agent of amoebic gill disease of Atlantic salmon (Salmo salar). Int J Parasitol. 2007; 37: 1469-1481.