CASE 2: 13-6752 (4048083-00)
Signalment: Two-month-old female Galloway cross calf.
History: This calf was from a group of about 100 Galloway and Galloway cross calves. One calf died 3 weeks previously but was not autopsied, and calves were treated for coccidia with sulfa boluses at that time. This calf had been sick for 1-2 days, exhibiting lethargy and dyspnea leading to lateral recumbency and death. The autopsy was performed shortly after death by the referring veterinarian.
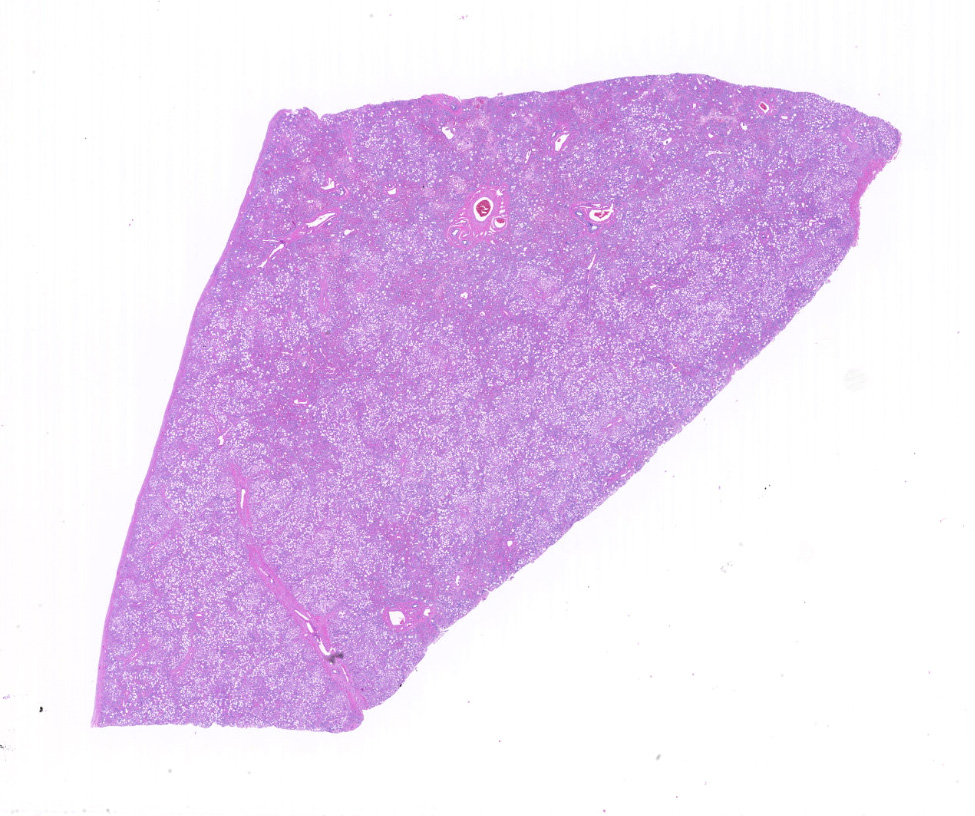
Gross Pathology: No icterus was noted. The liver and spleen were noted to be yellow-grey and did not bleed when cut. Only liver was submitted for histopathology.
Laboratory results: A postmortem blood sample was noted to have lipemic serum. PCV = 60%, total protein = 6.8. A fecal float was negative for parasite eggs.
Microscopic description:
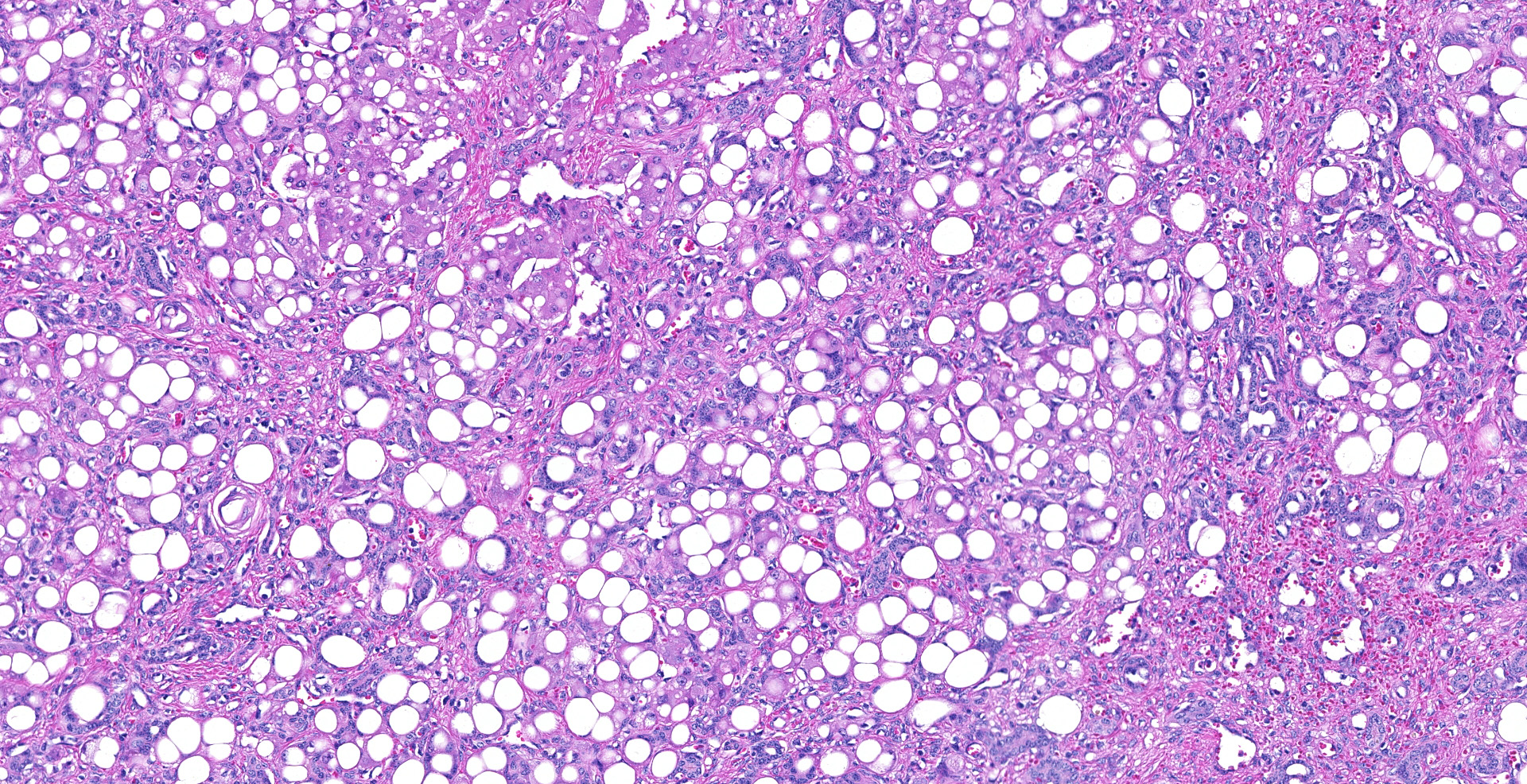
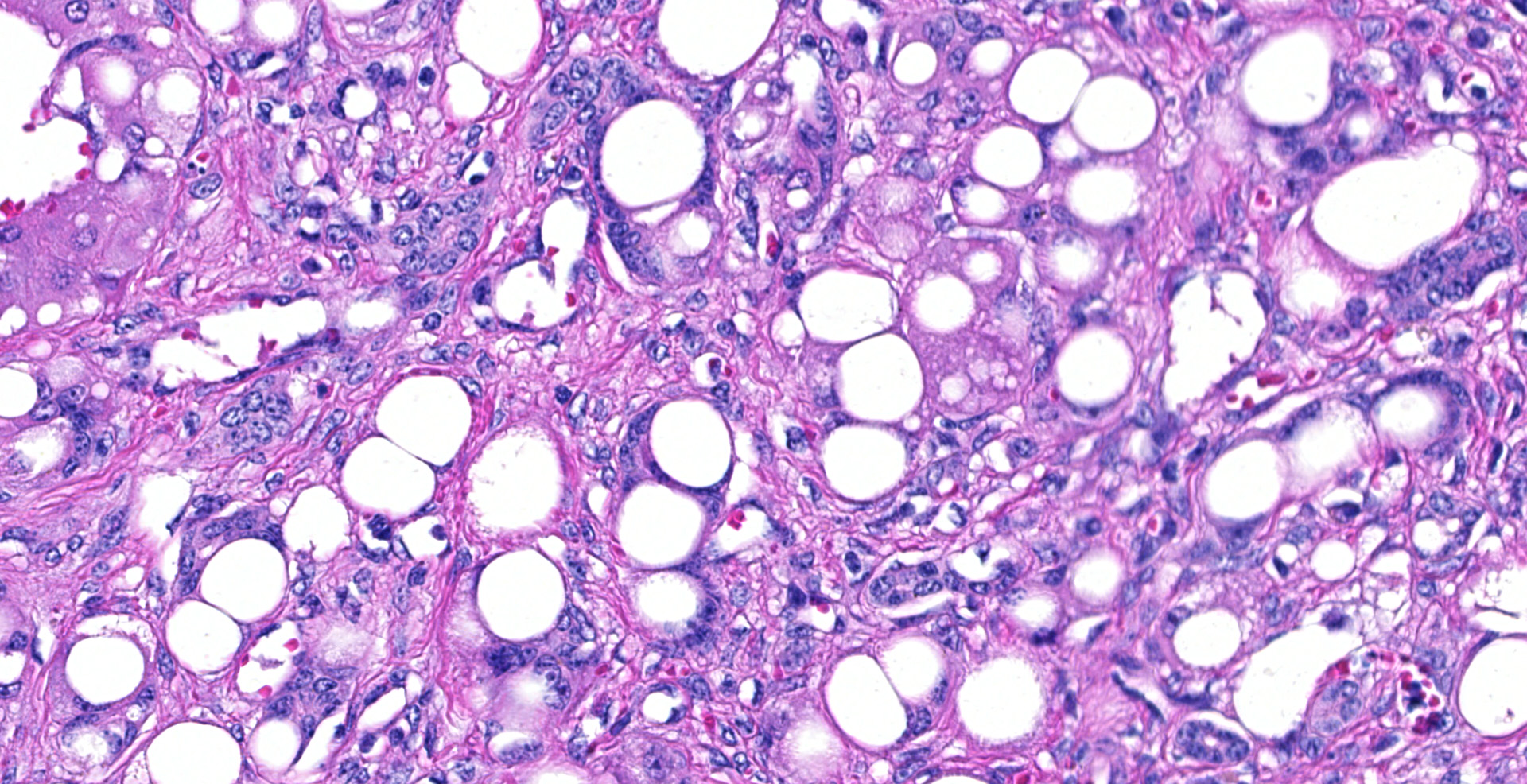
In representative sections of liver, the hepatic lobular organization and microanatomy were severely disrupted by fibrosis interspersed with bile ductules which dissected through all levels of the lobule and segregated the hepatocytes into variably sized nodules. The central veins were ill-defined and randomly located. The hepatocytes within the nodules were variably sized and contained large, clear, intracytoplasmic vacuoles that displaced the nucleus peripherally. Portal areas were markedly expanded by large amounts of fibrous connective tissue and multiple bile ductules, and the tunica media of the hepatic, portal and central veins were severely thickened by fibrous tissue with collapse of the vascular lumens. Hepatic sinusoids were tiny or absent and not contiguous.
Contributor's morphologic diagnosis:
Diffuse, severe, chronic hepatocellular lipidosis with severe periportal fibrosis and bile duct hyperplasia.
Contributor's comment:
Lesions were compatible with hepatic lipodystrophy of Galloway cattle. The condition has been diagnosed sporadically in the breed since 1965 but no genetic, metabolic or nutritional cause has been identified to date.6 In 1999, the clinicopathologic findings in 15 cases from 5 farms in Scotland were documented.4 Affected animals were usually normal at birth, although an aborted fetus and a stillborn calf had liver lesions similar to older affected calves. Within 2-4 months of birth calves became lethargic and progressed to tremors, seizures, recumbency and death. Significant clinical pathologic abnormalities included hypoalbuminemia, elevated liver enzymes and marked elevation in serum cholesterol, triglycerides and fatty acids. The only gross lesions noted at necropsy were large, pale livers. Histopathology of the liver was as described above; the only other histopathologic lesion was white matter spongiosis attributed to hepatic encephalopathy. No mention was made about the adequacy of fat stores in the bodies, which may be relevant to human conditions discussed below.
Hepatic lipidosis (or steatosis, in human medical terminology) refers to the excessive accumulation of triglycerides within hepatocytes.6 The liver's role in fat metabolism is complex and vital, and includes uptake of lipid from plasma chylomicrons, hydrolysis into fatty acids, repackaging via lipoprotein lipase and secretion of very low-density lipoproteins. Excessive storage of lipid as triglycerides in the liver can occur if the supply of lipid exceeds the liver's capacity to process, or if there is a disruption of one or more of the many metabolic steps involved in processing lipid through the peroxisomes and endoplasmic reticulum. In veterinary medicine, hepatic lipidosis can have a variety of pathogeneses, including physiologic (late pregnancy), metabolic (e.g. diabetes mellitus), toxic or nutritional (e.g. ovine white liver disease due to cobalt deficiency). Some conditions, such as ovine white liver, can progress to cirrhosis and liver failure.
In human medicine, lipodystrophies are a large group of rare genetic and acquired diseases characterized primarily by abnormalities in the amount and distribution of adipose tissue.5 Genetic forms are divided into congenital generalized lipodystrophy and familial partial lipodystrophy. The most common acquired form seen now is in HIV-positive patients treated with protease inhibitors as part of Highly Active Antiretroviral Therapy. Although partial or complete lack of body fat is the primary clinical sign, patients with congenital generalized lipodystrophy have several other clinical manifestations, including early onset hypertriglyceridemia and liver enlargement due to fat accumulation.2 Insulin resistant diabetes occurs early in life, but cirrhosis develops later. The protein systems defective in all of the reported human congenital and acquired lipodystrophies involve development or processing of lipid droplets within adipocytes.3 In none of the reviews is there mentioned a form of lipodystrophy with neonatal hepatic steatosis and cirrhosis.
While the disease in Galloway cattle may be a form of lipodystrophy, it does not appear to be a close correlate with any of the human syndromes. It may be more likely to be due to a defect in the liver's capacity to process and release lipids. Clearly more research is required to determine where this fascinating disease fits into the wide spectrum of defects in fat metabolism.
Contributing Institution:
Department of Veterinary Microbiology and Pathology
College of Veterinary Medicine
Washington State University
Pullman, WA 99164-7040
JPC diagnosis:
Liver: Hepatocellular lipidosis, diffuse, severe, with marked hepatocellular loss, fibrosis, and biliary reduplication, Galloway mix, bovine.
JPC comment:
The contributor succinctly illustrates the current understanding of this disease in Galloway cattle. There have been recent case reports in the United Kingdom7 and Germany, with increasing evidence of a genetic link. In the German herd, 7 calves sired by the same bull were affected, and the herd experienced no further occurrences when the bull was replaced.9
Congenital lipodystrophies encompass a variety of rare diseases associated with partial or total absence of normal adipose tissue. One variant, Berardinelli-Seip congenital lipodystrophy (BSCL) is autosomal recessive and has been linked to genetic mutations affecting a lipid biosynthetic enzyme 1-acyl-sn-glycerol 3-phosphate O-acyltransferase 2 (AGPAT2) or the integral endoplasmic reticulum membrane protein seipin. A strain of Agpat2 knockout mice have been created to further research of these diseases. At necropsy, Agpat2-/- mice are characterized by severe hepatomegaly, complete absence of white adipose tissue, amoeboid adipocytes with microvesiculated basophilic cytoplasm with deficient lipogenesis. Aggregates of brown adipose tissue are smaller and have massive necrosis and early ablation. These mice also have massive pancreatic islet hypertrophy, despite chronic hyperglycemia.8
While energy storage is a critical function of adipocytes, they also secrete a variety of hormones (adipokines) such as leptin, adiponectin, TNF-a, IL-6, resistin, and visfatin, all of which play necessary roles in regulating metabolism. With derangement of these adipokines, lipid metabolism becomes incomplete and animals fail to thrive.8
Feline hepatic steatosis, feline familial hyperlipoproteinemia, and canine primary idiopathic hyperlipidemia may be closer veterinary correlates to this disease in Galloway calves. The majority of pathologic changes are confined to the liver and involve various aspects of lipid processing ability of hepatocytes. While feline hepatic steatosis is common, the exact mechanism of hepatocellular accumulation of triglycerides remains incompletely described, with a likely multifactorial etiology. In feline familial hyperlipoproteinemia, there is an associated congenital lipoprotein lipase deficiency, leading to lipid vacuoles and ceroid accumulation in hepatocytes, as well as in the spleen, lymph nodes, kidneys, and adrenal glands.1
References:
- Cullen JM, Stalker MJ. Liver and Biliary System. In: Maxie MG ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Volume 2. 2016:277-278.
- Garg A, Agrawal A. Lipodystrophies: Disorders of adipose tissue biology. Biochim Biophys Acta 2009; 1791:507-513.
- Kramer N, Farese Jr RV, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med. 2013; 5:905-915.
- Macleod NSM, Allison CJ. Hepatic lipodystrophy of pedigree Galloway cattle. Vet Rec. 1999; 144:143-145.
- Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet 2014; 59:16-23.
- Stalker MJ, Hayes AM. Liver and biliary system In Pathology of Domestic Animals Fifth edition, ed. M Grant Maxie, Saunders/Elsevier, Edinburgh, 2007, Vol 2, pp 310-315.
7. Strugnell B, Wessels M, Woodger N, et al. Hepatic lipodystrophy of Galloway calves.. Vet Rec. 2015 Sep 12;177(10):265-6.
8. Vogel P, Read R, Hansen G, et al. Pathology of congenital generalized lipodystrophy in Agpat2-/- mice. Veterinary Pathology. 2011. 48(3):642-654.
- Weiland M, Mann S, Halfner-Marx A, Ignatius A. Hepatic lipodystrophy in Galloway calves. Veterinary Pathology. 2017;54(3):467-474.