Signalment:
5-year-old, male, mongrel, dog (
Canis familiaris).The dog presented with apathy, anorexia, vomiting, and diarrhea with blood, icterus (Figs. 1 and 2), fever
(40.8°C), mild dehydration, tachycardia, dyspnea and subcutaneous edema in the pelvic limbs and generalized
enlargement of the lymph nodes.
Gross Description:
There was marked yellow discoloration (icterus) of mucous membranes, skin, subcutaneous
tissues and intima of large arteries. The spleen was markedly (about 5x) enlarged (Fig. 5) and had a dry (no blood
oozing) fleshy texture to the cut surface (Fig. 6). All lymph nodes were moderately enlarged, soft, light brown and
wet at cut surfaces. The mucosa of the entire small intestine was dark red (hemorrhagic) and the contents were
admixed with blood. The lungs were red and wet and did not collapse when the thoracic cavity was opened (Fig. 7).
Large amounts of fluid oozed from the cut surface of the lungs. A large amount of whitish-pink foam could be
observed within the trachea and main bronchi (pulmonary edema). Additional findings included serous atrophy in
the coronary adipose tissue of the heart, hydropericardium, petechiae and paint brush hemorrhages in the
endocardium of the left ventricle. The bone marrow of the long bones was markedly red and filled the whole
marrow space.
Histopathologic Description:
Spleen and heart: In the spleen there is a marked inflammatory infiltrate consisting
of some lymphocytes and large numbers of plasma cells. The cellular infiltrate obliterates a large part of the splenic
red pulp. There are few plasmablasts and Mott cells within the inflammatory infiltrate; however, the majority of the
inflammatory cells consists of mature plasma cells. Multiple aggregates of histiocytes are seen throughout the
spleen and compress the adjacent splenic tissue. Oval to round, 2μm protozoal organisms can be observed within
the cytoplasm of endothelial cells of the splenic capillaries, but not in venules, arterioles, veins or arteries. There are
5-20 organisms per parasitized cell. In the liver (not submitted), there was paracentral coagulative zonal necrosis;
the cholangioles and bile ductules were distended by bile pigment and there was a lymphoplasmacytic inflammatory
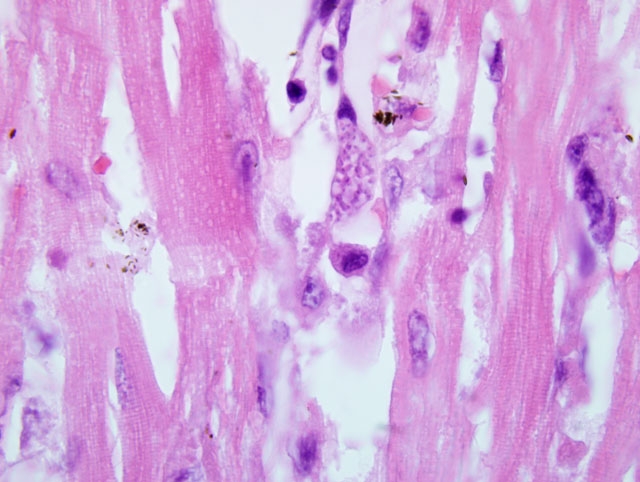
infiltrate in the portal triads. The same inflammatory infiltrate is observed in the myocardium, renal interstitium,
and pulmonary interalveolar septa. In the myocardium, the inflammatory infiltrate is associated with mild
degeneration of cardiomyocytes. Large numbers of nucleated RBCs occurred within hepatic sinusoids. The
protozoal organisms described in the spleen and myocardium can also be observed parasitizing endothelial cells of
capillaries in the kidney, lymph nodes, liver, bone marrow and choroid plexus of the fourth ventricle (Fig. 8).
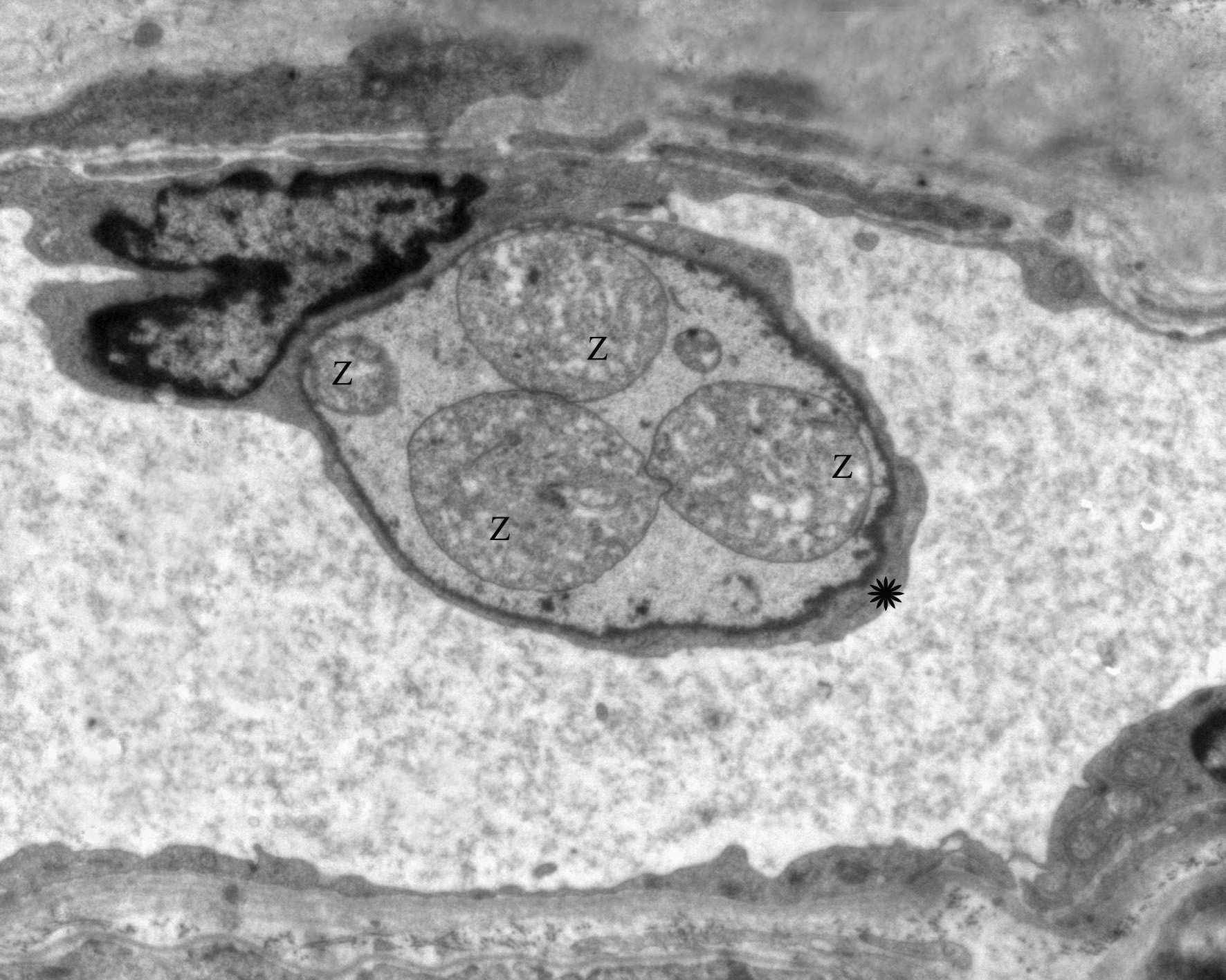
Transmission electron microscopy (TEM) of the choroid plexus (Fig. 9) shows zoites (z) within the cytoplasm
(asterisk) of an endothelial cell. Zoites (z) can also be visualized by TEM within endothelial cells of a lymph node
(Fig. 10).
Morphologic Diagnosis:
1) Spleen, reactive hyperplasia, lymphoplasmacytic, associated with
intraendothelial zoites, morphology consistent with
R. vitalii.Â
2) Myocardium, myocarditis, lymphoplasmacytic mild
to moderate, with mild fiber degeneration, associated with intraendothelial zoites, morphology consistent with
R.
vitalii.
Lab Results:
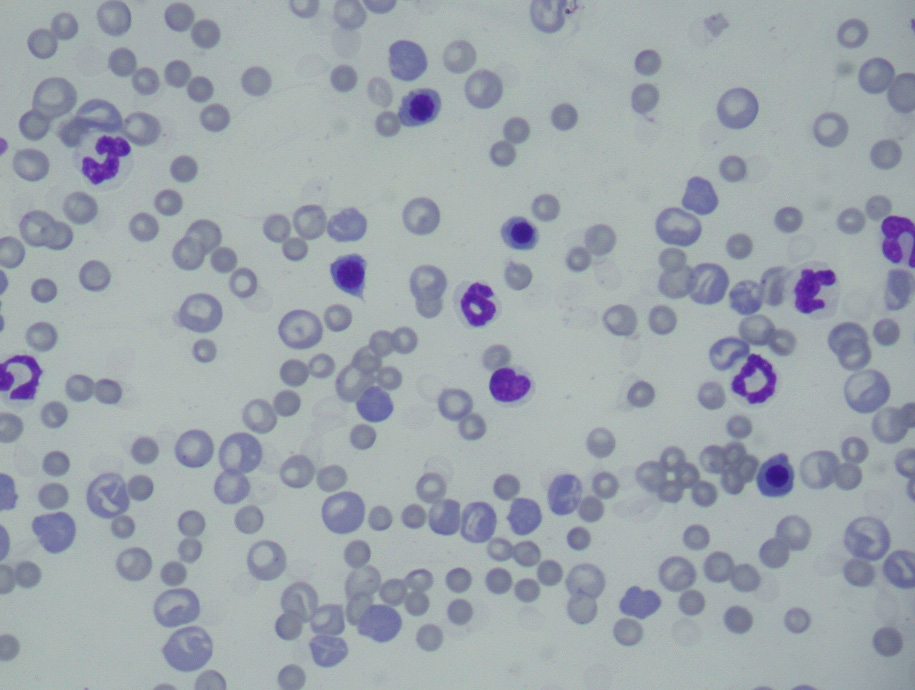
A CBC performed at presentation revealed hypochromic macrocytic regenerative anemia,
leucocytosis due to regenerative left shift and lymphocytosis, and regenerative thrombocytopenia (Table 1). Blood
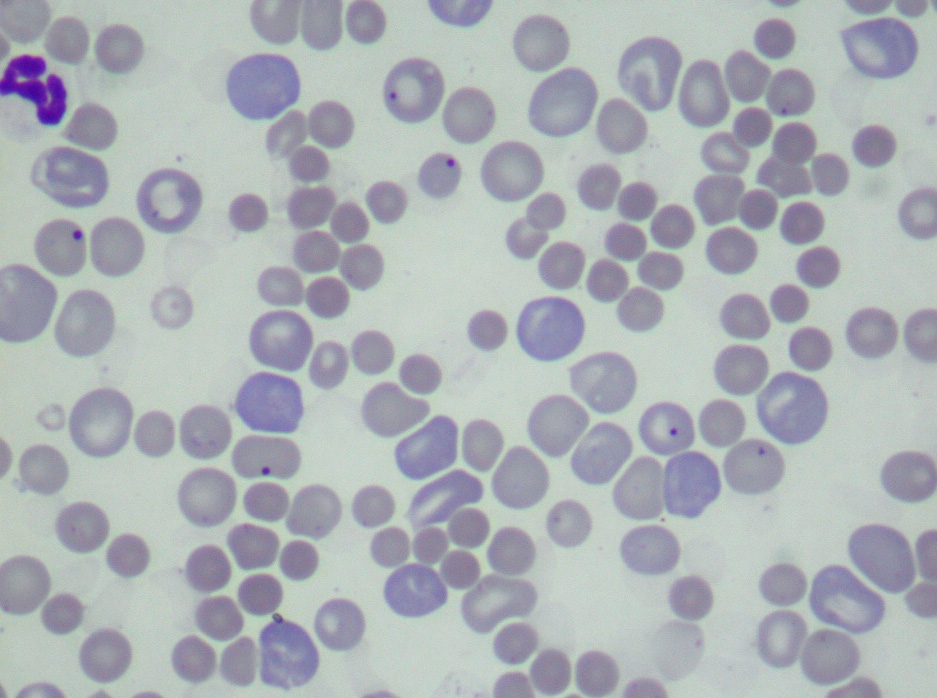
smears revealed marked anisocytosis and polychromasia, several RBCs with Howell-Jolly bodies (Fig. 3), and large
numbers of nucleated RBCs, mainly metarubricytes (23/100 leucocytes), but also lesser numbers of rubricytes
(2/100 leucocytes) (Fig. 4). Marked spherocytosis (Fig. 3) and large platelets (macroplatelets) were additional
findings in the blood smear. No blood parasites were found neither within blood cells nor free in the plasma.
The biochemistry panels showed mild increase in total plasma proteins due to increase in albumin, moderate
increase in serum activity of alanine aminotransferase and marked increase in the total serum bilirubin (Table 2).
Urinalysis revealed marked bilirubinuria.
Based on the laboratory results described above, a clinical diagnosis of extravascular hemolytic anemia was
established. The marked spherocytosis suggested an immune mediated origin. The dog was treated with 1 mg/kg
prednisone but died the following day.
Table 1 - CBC (reference values are within parentheses)
| WBCs (/mm3) | 32200 | (6,000-17.000) | RBCs (x106/mm3) | 1.3 | (5.5-8.5) |
| Neutrophils (%) | 72 | (60%-77%) | | | |
| Neutrophils (abs.) | 23184 | (3,000-11,500) | | | |
| Bands (%) | 6 | (0%-3%) | Hemoglobin (g/dL) | 3.8 | (12.0-18.0) |
| Bands (abs.) | 1932 | (0-300) | | | |
| Metamyelocytes (%) | 2 | (0%) | | | |
| Metamyelocytes (abs.) | 644 | 0 | Hematocrit (%) | 12 | (37-55) |
| Myelocytes (%) | - | (0%) | | | |
| Myelocytes (abs.) | - | 0 | | | |
| Lymphocytes (%) | 17 | (12%-30%) | MCV (fl) | 92.3 | (60.0-77.0) |
| Lymphocytes (abs.) | 5474 | (1,000-4,800) | | | |
| Monocytes (%) | 2 | (3%-10%) | | | |
| Monocytes (abs.) | 644 | (150-1,350) | MCHC (%) | 31.7 | (32.0-36.0) |
| Eosinophils (%) | 1 | (2%-10%) | | | |
| Eosinophils (abs.) | 322 | (100-1,250) | | | |
| Basophils (%) | - | (rare) | Platelets (x103/mm3) | 98 | (200-500) |
| Basophils (abs.) | - | (rare) | | | |
Table 2 - Biochemistry panel
| Parameter | Unit | Result | Reference values |
| Alanine aminotransferase | U/L | 180 | 4.0-24.0 |
| Albumin | g/dL | 4.4 | 2.6-3.3 |
| Creatinine | mg/dL | 1.2 | 0.5-1.5 |
| Total bilirubin | mg/dL | 6.5 | 0.1-0.5 |
| Fibrinogen | mg/dL | 200 | 200-400 |
| Globulins | g/dL | 4.2 | 2.7-4.4 |
| Total plasm proteins | g/dL | 8.8 | 6.0-8.0 |
| BUN | mg/dL | 56.0 | 21.0-60.0 |
Condition: Rangelia vitalii
Contributor Comment:
Based up on the clinicopathological findings, a diagnosis of hemolytic anemia
associated with infection by Rangelia vitalii (rangeliosis) was made. Rangeliosis, colloquially known as nambi-uv+�°
(in native Brazilian Indian idiom meaning literally bleeding ear), peste de sangue and febre amarela dos
c+�-�es (Portuguese for blood ill and yellow fever of dogs, respectively) is an extravascular hemolytic disorder
affecting dogs from southern Brazil. For several reasons this disease remained almost forgotten for the last 50 years,
and during this period it was variably mistakenly diagnosed as other canine infectious diseases (hemolytic or
otherwise), such as babesiosis, erlichiosis and leishmaniasis. The true nature of the disease surfaced again in 2001
when a group of Brazilian researchers put forth an effort to elucidate its cause, pathogenesis and etiology, and thus
established it as a distinct disease entity of dogs. (3,4,6) Although the precise taxonomic classification of the causative
organism of rangeliosis is still uncertain, the agent was established as a protozoal organism of the phylum
Apicomplexa, order Piroplasmorida based on ultrastructural, immunohistochemical and in situ hybridization studies.
(5) Until definitive nomenclature is established, Rangelia vitalii (named after two Brazilian researchers from the
first half of the 20th Century - Rangel and Vital ) is maintained as the parasites designation.
It is currently accepted that R. vitalii is transmitted by tick vectors (Rhipicephalus sanguineus and Amblyomma
aureolatum) to dogs and several wild mammal species in the State of Rio Grande do Sul (RS) in southern Brazil.
The life cycle of R. vitalii is unknown, but it has been speculated that the vectors R. sanguineus and A. aureolatum
circulate the protozoan between wild mammals and domestic dogs, the latter probably being an aberrant host.(3)
Experimental transmission to susceptible dogs using blood from spontaneously affected dogs was achieved recently
in two independent studies.(4,5) The clinical and laboratory aspects of experimental disease differ somewhat from
natural disease, suggesting that the parasite needs to replicate in the tick in order to acquire some aspects of
virulence. Experimental disease is also fatal if left untreated.
The great majority of dogs with spontaneous rangeliosis develop clinical signs of extravascular hemolysis: pallor of
mucous membranes; icterus; and hepatosplenomegaly.(3,4) Other clinical signs include apathy, anorexia, fever,
vomiting, diarrhea, mucopurulent oculonasal discharge, tachypnea, tachycardia, subcutaneous edema of the pelvic
limbs, and petechiae and ecchymosis on the mucous membranes.(3)
Hematologic findings are typical of extravascular hemolysis and include hypochromic macrocytic anemia with
excessive regeneration. Anisocytosis, polychromasia, Howell-Jolly bodies and several nucleated red blood cell
precursors (metarubricytes and rubricytes) are observed on peripheral blood smears.(3,4) Most of the affected dogs
also present varying degrees of spherocytosis,(3,4) a hematological finding highly suggestive of immune mediated
hemolytic anemia.(1) The numbers of circulating reticulocytes are high (5%-28%, average 12,5%).
Erythrophagocytosis is occasionally observed, particularly in those cases in which there is marked associated
spherocytosis. Normochromic normocytic anemia is observed on occasion due to the extreme contrast between the
small and falsely hypochromic-appearing spherocytes and the large polychromatophils recently released from the
bone marrow. Plasma from affected dogs is bright yellow. WBC counts frequently reveals leucocytosis due to
regenerative left shift resulting from long-standing and non-specific stimulation on the bone marrow. In some of the
cases the leucocytosis may present as a leukemoid reaction. Other common hematological findings include
lymphocytosis and monocytosis.(3)
In a smaller percentage of the cases of spontaneous canine rangeliosis, affected dogs develop a bleeding disorder
similar to DIC characterized by extensive hemorrhage from the tips, margins, and outer surface of the pinnae.(5) Dogs
affected in this manner have a moderate decrease in platelet numbers; numerous large platelets are observed in the
circulation, indicating regenerative thrombocytopenia. However, due to its moderate intensity, it is apparent that
thrombocytopenia itself is insufficient to induce the hemorrhage in these dogs, and some other disorder(s) of
hemostasis may be in place.(3,4)
There is no specific biochemical test for the diagnosis of rangeliosis, but serum alanine aminotransferase (ALT) is
increased in most cases. This elevation most likely results from anemia, hypoxia of centrolobular hepatocytes, and
death of these cells. Bilirubin levels are consistently increased due to the extravascular hemolysis and the urine is
darkened by the excretion of large amounts of bilirubin and urobilinogen; hemoglobinuria is never part of the
clinical picture.(3)
In contrast to most infectious hemolytic anemias, the clinical diagnosis of rangeliosis is generally made on response
to therapy, since the parasite is seldom found in the blood smears from affected dogs (only in approximately 4% of
the cases).(3) Currently, there is no commercial or in-house tests to detect antibodies or antigens associated with R.
vitalii. Fine needle aspiration (FNA) or excisional biopsy of lymph nodes, spleen and bone marrow with subsequent
cytological evaluation can also be important aids in the clinical diagnosis. Due to the intraendothelial location of the
parasite, fine needle aspiration may yield false negative results.
Necropsy findings in dogs dying from rangeliosis are consistent with those seen in other causes of extravascular
hemolysis. The mucous membranes, subcutaneous tissue, muscle fascia, serosal surfaces and arterial intima are
markedly icteric and peripheral blood is thin and watery. The liver is enlarged and has a red-orange hue or, in more
severe cases, a greenish discoloration. Accentuation of the hepatic lobular pattern is common. The spleen is
enlarged and fleshy and all lymph nodes are swollen, moist, and red,(3) and may have multifocal to coalescing white
areas.(5) Hyperplastic bone marrow is markedly red and fills the entire marrow space.
Microscopic evaluation of lymph nodes reveals marked erythrophagocytosis and, depending on the stage of the
disease, there is hemosiderosis and severe paracortical lymphoid hyperplasia. In the spleen, there is lymphoid
hyperplasia characterized by marked plasmacytosis which, in some cases, resembles the plasma cell proliferation
pattern observed in myelomas; however, in rangeliosis, most plasma cells are mature and there are few plasmablasts,
Mott cells and/or flame cells.(3)
In non-lymphoid organs, the same lymphoplasmacytic infiltrates are observed, and occasionally there is a
granulomatous infiltrate which includes multinucleate giant cells that sometimes phagocytize parasitized endothelial
cells.(4) In the liver, there is centrolibular coagulative necrosis and accumulation of bile pigment. In the bone
marrow, there is marked erythroid and megakaryocytic hyperplasia characterized by replacement of the marrow
adipose tissue by hematopoietic cells with high mitotic index, a drop in the myeloid to erythroid ratio, and increased
numbers of megakaryocytes and megakaryoblasts. Foci of extramedullary hematopoiesis can be observed
frequently in the spleen and liver and less frequently in the lymph nodes and adrenal glands.(3)
The parasite is found within the cytoplasm of endothelial cells in several organs and consists of 2.0-2.5 μm round to
oval, basophilic organism when stained with hematoxylin and eosin; its cytoplasm is pale and inconspicuous and the
nucleus is prominent, basophilic and eccentrically located.3,5 The frequency in which the parasite was observed in
several organs was reported from the study of 11 cases of canine rangeliosis as: kidneys (7/11), lymph nodes (7/11),
liver (6/11), bone marrow (4/11), spleen (3/11), tonsils (2/11), lung, brain, stomach and gallbladder (1/11).(4) The
authors pointed out that not all organs were histologically examined in each of the 11 cases. Other reports indicate
lymph nodes, bone marrow, kidneys and choroid plexus are the organs with the highest parasite load.(4) About 20-30
organisms can be found within the cytoplasm of each parasitized endothelial cell. The organisms usually can be
seen in smears from bone marrow sampled during the necropsy and stained with Giemsa and Panoptic.(4) In various
reports, immunostaining for Leishmania chagasi, Neospora caninum and Toxoplasma gondii was consistently
negative;(4,5) however, the parasite reacted positively with an anti-Babesia microti antibody.(5) R. vitalii was also
positive on in situ hybridization for B. microti(4) indicating that the organism belongs to the same group as Babesia
However, unlike babesia R. vitalii is characterized by an intraendothelial stage. Parasitized cells are immunopositive
for Willebrand factor, indicating their endothelial nature. The ultrastructural characteristics of R. vitalii were
reported by Loretti & Barros 2005 (these authors mistakenly spelled the parasites name as R. vitalli) as having an
apical complex that includes a polar ring and rhoptries but no conoid; the parasite is contained within a
parasitophorous vacuole that had a trilaminar membrane with villar protrusions and was located within the
cytoplasm of capillary endothelial cells.(6)
Necropsy finding of dogs dying from rangeliosis, coupled with the hematological findings, suggest the diagnosis of
hemolytic anemia; the finding of the causative agent in the cytoplasm of endothelial cells confirms a presumptive
diagnosis.
Differentials for this case should include infection by R. vitalii, Histoplasma capsulatum, Trypanosoma cruzi,
Leishmania spp., Toxoplasma gondii, and Neospora caninum. Histoplasma and Leishmania organisms are typically
found in macrophages and may elicit an intense histiocytic to granulomatous response. H. capsulatum yeasts stain
with PAS and GMS stains. Leishmania and T. cruzi have a kinetoplast, which is absent in R. vitalii. Additionally,
the amastigotes of T. cruzi form pseudocysts within the sarcoplasm of cardiomyocytes and not within endothelial
cells. The zoites of T. gondii and N. caninum form 2-5 μm and 4-7 μm tachyzoites, respectively, and have no
kinetoplast. N. caninum can parasitize endothelial cells but usually parasitize a large spectrum of host cells and are
PAS positive. Similarly, T. gondii affects a large spectrum of host cells, although usually not endothelial cells, and
induces a whole different set of lesions.
JPC Diagnosis:
1. Heart: Myocarditis, lymphoplasmacytic and histiocytic, multifocal, mild to moderate with
cardiomyocyte degeneration, capillary fibrin thrombi and numerous intraendothelial protozoa.
2. Spleen: Plasmacytosis, diffuse, marked with multifocal reticuloendothelial cell hyperplasia and scattered
intraendothelial protozoa.
Conference Comment:
The contributor provides an excellent, thorough discussion of this unique and poorlyknown
infection. Conference participants were unfamiliar with this condition. Many favored T. cruzi. The key
observation necessary to reach the correct diagnosis is the intraendothelial location of the organism. Once that is
recognized, internet searches could lead to articles on this fascinating disease.
While reviewing the submitted CBC, biochemistry panel and cytology, conference participants discussed the
clinicopathologic differentiation between extravascular and intravascular hemolysis. The diagnostic features for
each are briefly summarized in the chart below.(2)
| | Extravascular Hemolysis | Intravascular Hemolysis |
| Cause (general categories) |
1. Antibody and/or complement mediated
2. Decreased RBC deformability
3. Premature RBC aging (decreased glycolysis
and [ATP])
4. Increased macrophage phagocytosis |
1. Complement-mediated lysis
2. Physical damage
3. Oxidative damage
4. Osmotic lysis
5. Membrane alterations
|
| Onset | Usually chronic course with insidious onset | Peracute to acute |
| CBC | Neutrophilia, monocytosis, thrombocytosis | No significant findings |
| Biochemistry | Hyperbilirubinemia with the unconjugated
form dominating early |
1. Hemoglobinemia: plasma discolored red;
increased MCHC and MCH; decreased serum
haptoglobin
2. Hyperbilirubinemia with unconjugated
dominating early |
| Urinalysis | Bilirubinuria | Hemoglobinuria; hemosiderinuria; -�
Bilirubinuria |
| Erythrocyte
morphology |
Spherocytes, schistocytes, keratocytes; if a
regenerative response, reticulocytes, Howell-
Jolly bodies, polychromasia and macrocytosis |
Schistocytes, keratocytes, Heinz bodies,
eccentrocytes |
Certain breeds of dogs and cats possess hereditary biochemical deficiencies or predispositions to extravascular
hemolysis.(2) In dogs, phosphofructokinase deficiency is seen in cocker spaniels, English springer spaniels and some
mixed breeds. Pyruvate kinase deficiency is noted to occur in the Basenji, beagle, Chihuahua, dachshund, pug,
miniature poodle, West Highland White terrier, and Cairn terriers as well as Abyssinian, Somali and domestic shorthaired
cats. A hereditary predisposition to hemolytic anemia is suspected in the border collie, cocker spaniel,
English springer spaniel, German shepherd dog, Irish setter, Old English sheepdog, poodle and the whippet.
References:
1. Barker RN. Anemia associated with immune responses. In: Feldman BF, Zinkl JG, Jain NC, eds. Schalms
Veterinary Hematology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins: 2000:169-177.
2. Brockus CW, Andreasen CB. Erythrocytes. In: Latimer KS, Mahaffey EA, Prasse KW, eds. Duncan and Prasses
Veterinary Laboratory Medicine: Clinical Pathology. 4th ed. Ames, IA: Blackwell Publishing; 2003:26-38.
3. Fighera RA: Rangeliose. Acta Scientiae Veterinariae 2007;35(Supl2):264-266.
4. Krauspenhar C, Fighera RA, Gra+�-�a DL. Anemia hemol+�-�tica em c+�-�es associada a protozo+�-�rios. MEDVEP
2008;1:273-281.
5. Loretti A, Barros SS. Hemorrhagic disease in dogs infected with an unclassified intraendothelial piroplasm in
southern Brazil. Vet Parasitol. 2005;134:193-213.
6. Loretti A, Barros SS: Infec+�-�+�-�o por Rangelia vitalli (Nambiuv+�°, Peste de Sangue) em caninos: revis+�-�o.
MEDVEP. 2004;2:128-144.

Click the slide to view.
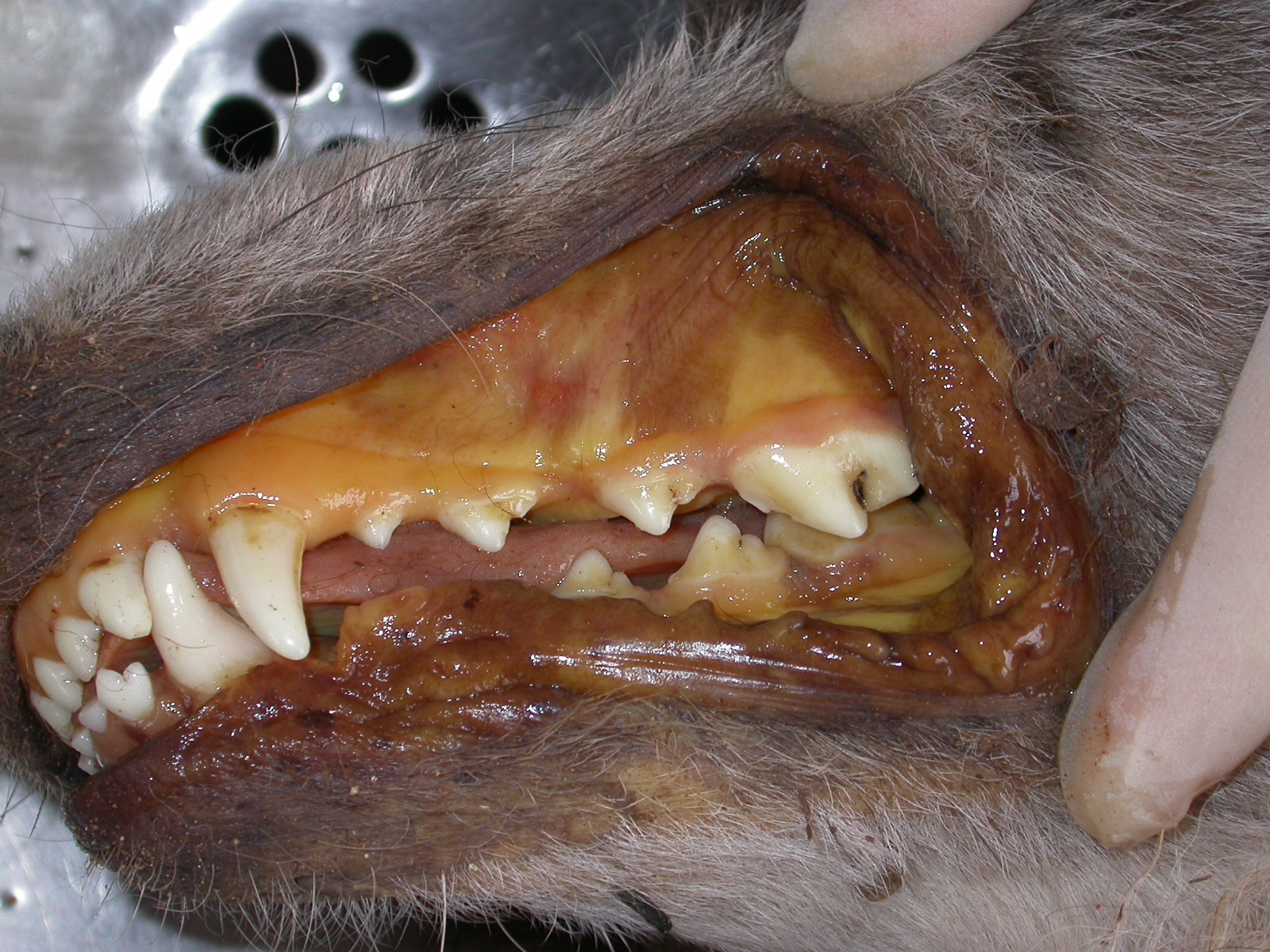
4-1. Oral mucosa
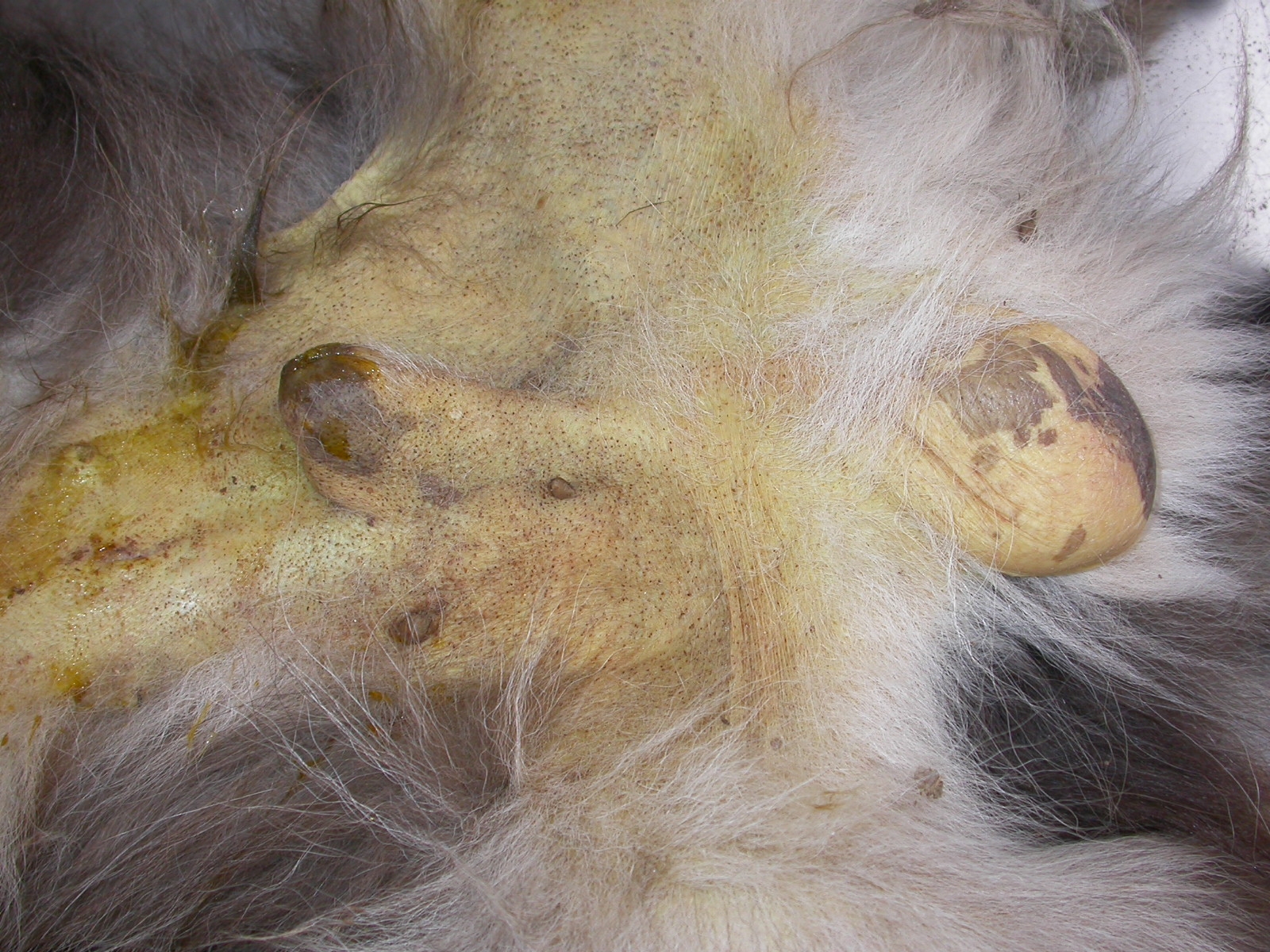
4-2. Skin
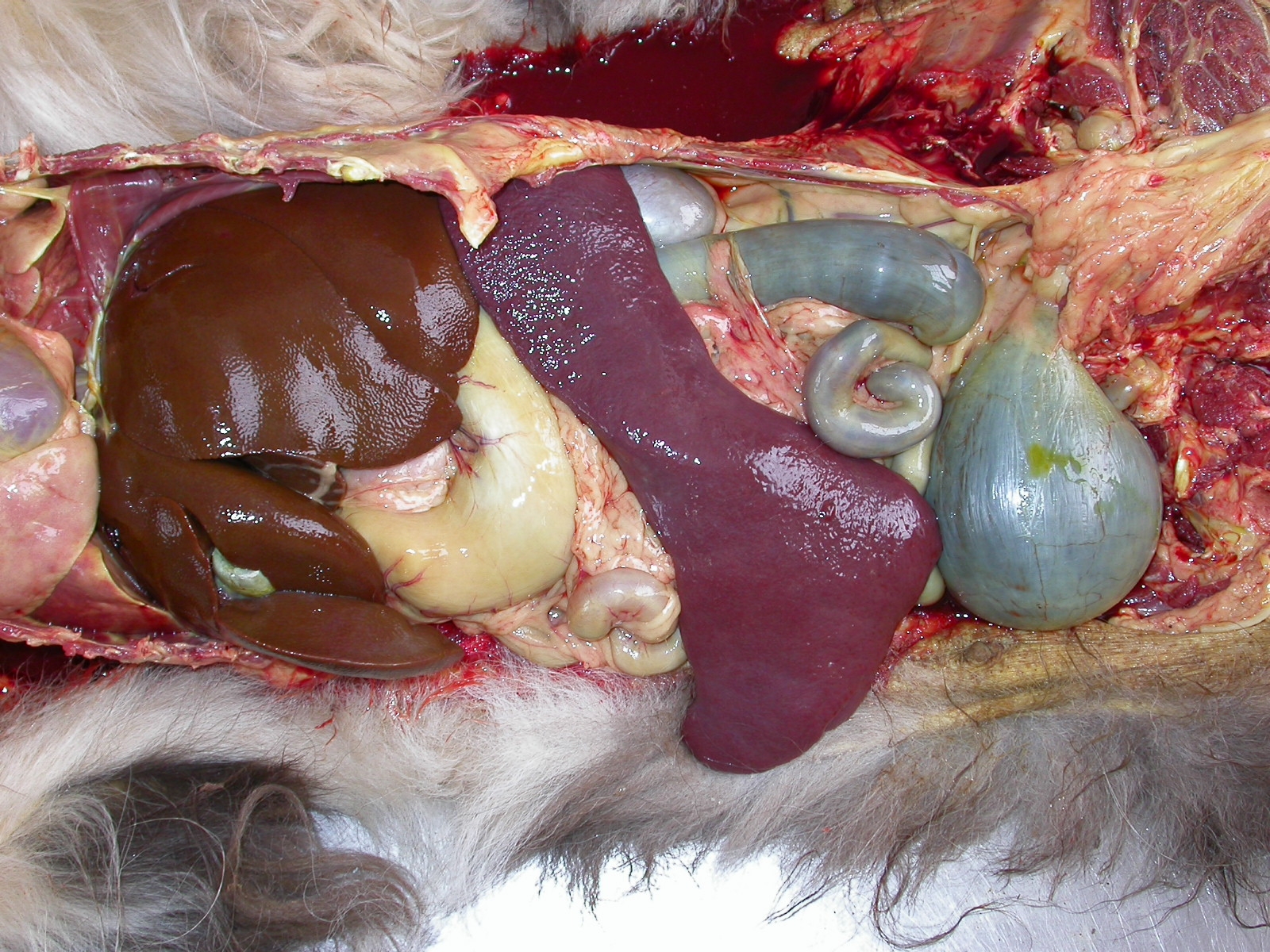
4-3. Spleen

4-4. Spleen
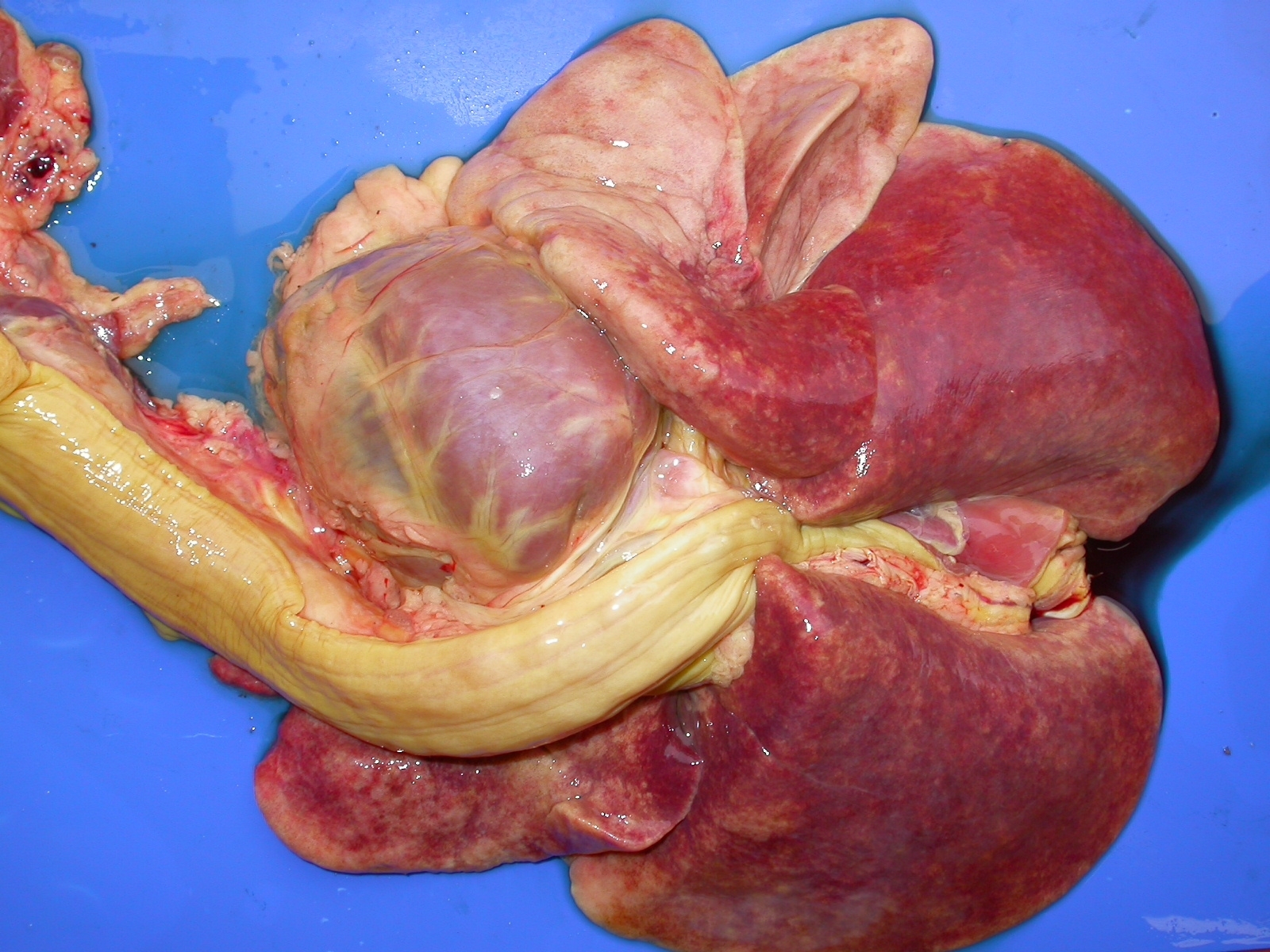
4-5. Heart and lung
4-6. Peripheral blood
4-7. Peripheral blood
4-8. Heart

4-8. Heart
4-9. Choroid plexus, endothelial cell
| Back | VP Home |
Contact Us |








