WSC 32023-2024 Conference 2, Case 3
Signalment:
11-month-old, female spayed Labrador Retriever dog, canine (Canis lupus familiaris)
History:
The dog submitted to the CHUV (Centre Hospitalier Universitaire Vétérinaire), Faculté de Médecine Vétérinaire, University of Montreal, because of recurring respiratory difficulties (panting with its mouth closed) along with a head tilt and turning to the right. Clinical signs had been ongoing for over 9 months. Clinical signs were still present after therapeutic trials with different immunosuppressive drugs. Humane euthanasia was performed at home by the family veterinarian due to the guarded prognosis.
Gross Pathology:
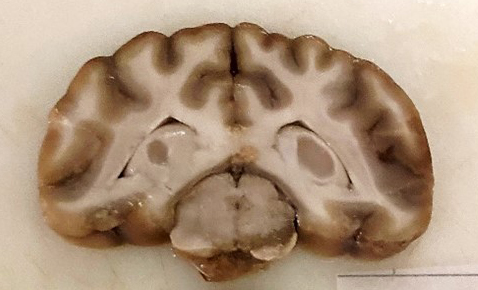
This 24.5 kg Labrador Retriever dog is submitted frozen for necropsy examination and is in a suboptimal state of conservation following thawing. The animal has adequate muscle mass and body fat stores. The right portion of the cerebral trunk displays a 3x1x1cm area of greyish discoloration which extends slightly to the left of the midline. The overlying leptomeninges have a dark discoloration. The spinal cord is unremarkable grossly. The heart weighs 221 g (0.9% of BW, within normal limits). All other internal organs are normal grossly.
Microscopic Description:
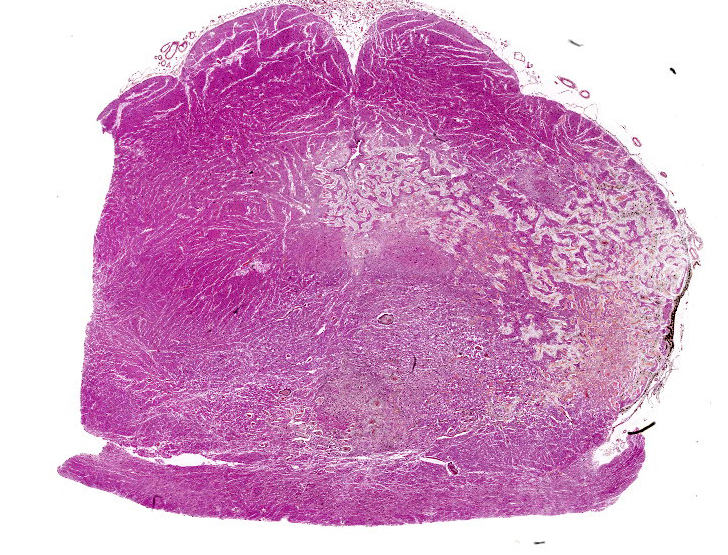
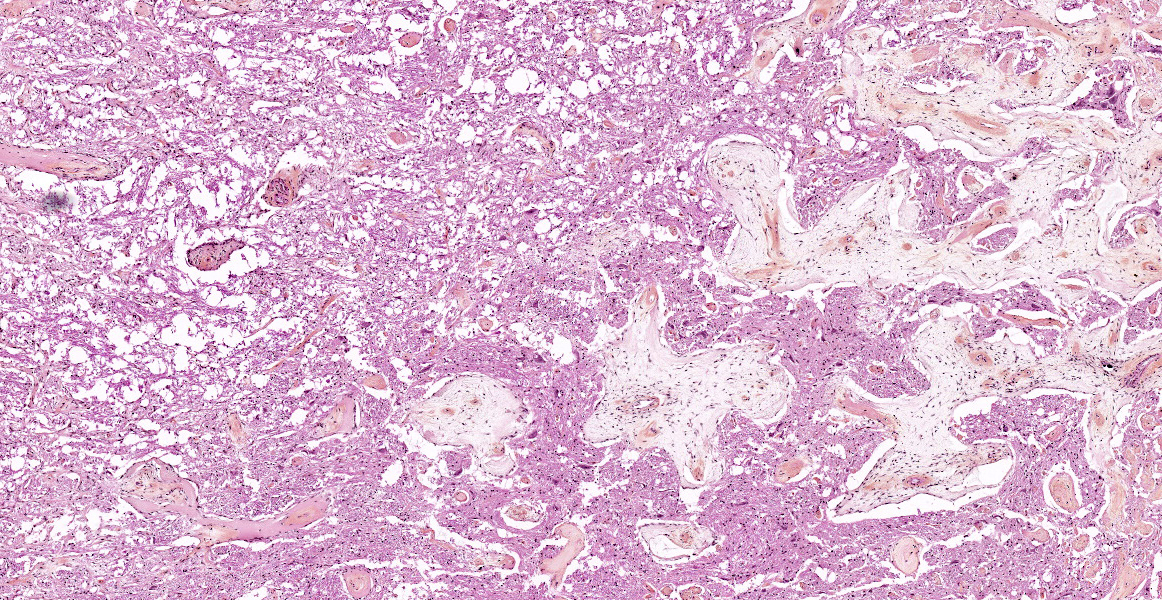
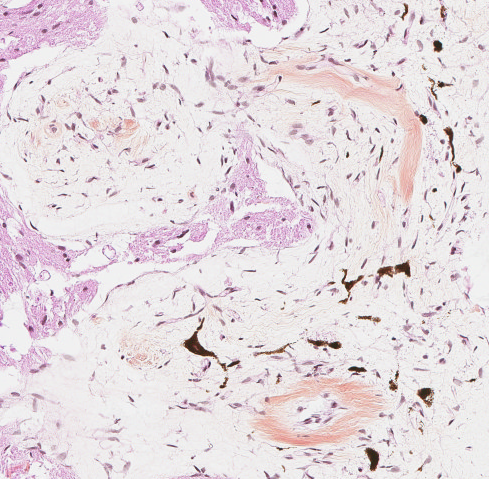
Brain: The observed gross changes in the area of the brain stem correspond to a poorly cellular and poorly delineated process that is continuous with the leptomeninges and multifocally infiltrates the neuroparenchyma. These changes are characterized by anastomosing fronds of spindle to stellate cells surrounded by a mostly loosely arranged fibrous stroma of variable density. This stroma is usually centered around numerous blood vessels, mostly small arterioles. The fusiform cells have a pale acidophilic cytoplasm that is finely fibrillar and moderately well delineated. The nucleus varies from elongated with rounded ends to round with a finely granular chromatin with no apparent nucleolus. Anisocytosis and anisokaryosis are minimal, atypia is unremarkable and no mitoses are observed. These spindle cells do not stain with GFAP immunohistochemistry staining. The lining of blood vessels located within that stroma is positive for Factor VIII. The leptomeninges overlying these proliferative changes are mildly to moderately thickened multifocally in some areas by similar proliferating spindle cells and contain multifocal and superficially numerous melanin-laden cells (melanocytes). In the adjacent neuroparenchyma, few neurons displaying chromatolysis and rare spheroids are observed.
Contributor’s Morphologic Diagnosis:
Brain stem: Cerebral meningioangiomatosis
Contributor’s Comment:
Meningioangiomatosis (MA) is, in animals at least, a rare benign lesion, best regarded as a malformation or hamartoma producing circumscribed plaques on the surface of the brain stem and cervical spinal cord.3 Hamartomas are mass lesions characterized by disorderly overgrowth of tissue elements. MA is seen most commonly in young dogs. Blood vessels appear in excess in these lesions and are cuffed by proliferating cells considered meningothelial in origin. The lesion usually does not extend into the underlying neural substance, which shows mixed degeneration and reactive changes, but can occasionally grow deeper along perivascular spaces.3,19
In veterinary medicine, this unique lesion has been described in a few dogs, one cat, one horse with associated mushroom toxicity, one cow that was part of a neuropathology BSE survey in Scotland, and in a CD-1 mouse. 1,2,4,10,15,17,21,22,25 This lesion described by Corbett et al in a 13-y-old cat was located in the leptomeninges and outer neuroparenchyma of the right pyriform and temporal telencephalic lobes, with extensive hemorrhage.4
Human MA has been described as coexisting with meningiomas, arteriovenous malformations, oligodendrogliomas, meningeal hemangiopericytomas and orbital erosions.7 An association with another nervous lesion has also been described in a 3-month-old German shepherd dog with a thalamic astrocytic hamartoma with tectal meningioangiomatosis, and in a 4-y-old Labrador retriever dog with a fibrous meningioma.11,23
MA typically occurs in the brain stem and cervical spinal cord of young dogs. An imaging study documented thoracolumbar spinal cord involvement in two dogs (4-y-old male Boxer and 5-month-old female Labrador), an unusual site for this condition.12 In a 5-year-old dog, a focus of MA located in the caudal thoracolumbar spinal cord and associated with abnormal hind limb gait was successfully excised and resulted in improvement of clinical signs with a good long-term prognosis.6 This benign lesion could then be curable with surgical resection depending on accessibility of the location, as in humans.9
In humans, the sporadic form of MA often results in headache and epilepsy. Focal cortical dysplasia has been described adjacent to foci of such lesions following surgical resection in cases of the sporadic form.13 MA lesions can demonstrate variable degrees of calcification, cystic degeneration, tumor-like appearance and/or enhancement, making radiologic diagnosis a challenge. Multicystic MA is also recognized as a rare variant of the condition, in which the cysts may have resulted from the gradual accumulation of cerebrospinal fluid in the perivascular spaces of arachnoid/vascular tissue trapped in the cortical parenchyma.16 Diagnosis of MA lesions can prove difficult but if done timely, prognosis with adequate surgical resection is typically good for seizure control in humans.9,18,26
The other form of MA is, in humans, associated with neurofibromatosis type 2 (NF2). NF2-associated lesions are usually asymptomatic. The autosomal dominant mutation in NF2 in humans is located on chromosome 22q12 and encodes for the protein merlin, which is widely expressed and important for cell growth regulation. Individuals with this mutation have a predisposition to develop tumors, particularly vestibular schwannomas and meningiomas, amongst others.5,26 MA and associated meningioma tissues were evaluated in a case series of 5 human patients using a next generation sequencing assay targeting 1425 cancer-related genes. Of the two MA cases associated with meningioma, one had deletions in the NF2 gene in both the MA and the meningioma, whereas the other had a NF2 deletion in only the MA component. Additional mutations were identified in the MA components of these cases, suggesting that the MA arises from the meningioma rather than the opposite.8
In another small case series involving six human patients with MA, neurofibrillary tangles were observed in neurons present both within the MA plaques and in the surrounding cortex. Neither senile plaques nor granulovacuolar degeneration were noted. The factors stimulating the production of neurofibrillary tangles in these cases remains unknown.14
Contributing Institution:
Centre de Diagnostic Vétérinaire de l’Université de Montréal (CDVUM)
Faculté de Médecine Vétérinaire
Université de Montréal
Saint-Hyacinthe, Qc, Canada
cdvum@umontreal.ca
JPC Diagnosis:
Brainstem: Meningioangiomatosis
JPC Comment:
The contributor provides an excellent overview of meningioangiomatosis (MA), a rare entity in both human and veterinary medicine. Being our second hamartomatous entity of the week, it also provides an opportunity to compare and contrast the pathogeneses of NF2-associated MA and the dysplastic cerebellar gangliocytoma examined in the previous case.
Both entities are caused by loss of function mutations in tumor suppressor genes: PTEN in the case of dysplastic cerebellar gangliocytoma, and NF2 in certain cases of MA. NF2 encodes the protein product merlin, which is expressed primarily in neural tissue and mediates contact inhibition of cell growth via signals received from the extracellular matrix.20 Merlin performs its wizardry as a cell proliferation/arrest toggle, signaling cell proliferation pathways when phosphorylated and cell growth arrest pathways when hypo-phosphorylated. At high cell density, merlin becomes hypo-phosphorylated in response to hyaluronate (HA), a component of extracellular matrix that surrounds cells, thus preventing proliferation when the cellular neighborhood becomes too crowded. Merlin’s pro-growth activity is mediated by a cytoplasmic interaction with CD44, a transmembrane HA receptor; when cell density is low, merlin is phosphorylated, complexed with CD44, and growth permissive.20
Similar to PTEN in the previous case, merlin operates in a complex signaling environment. When not complexed with CD44, merlin is active and inhibits receptor tyrosine kinases (RTKs) and their downstream targets, including the previously discussed PI3K/AKT pro-growth pathway. In this way, active merlin functions similarly to PTEN, but at an upstream point in the signaling cascade.24 In this state, merlin also simultaneously signals pro-apoptotic pathways and inhibits survival and growth pathways. Once complexed with CD44, however, merlin is phosphorylated, inactivated, and no longer able to inhibit RTKs, leading to the reverse effects described above: the PI3K/AKT pathway is activated, apoptotic pathways are inhibited, and survival and proliferative pathways are activated.24 In cases of NF2-associated MA, the mutation in NF2 leads to a dysfunctional merlin, which is unable to function as a brake on the RTK, PI3K/AKT cell growth pathway, leading to a variety of neuroproliferative conditions, including MA.
Signaling pathways are complex webs, with redundant, competing inhibitory and proliferative signals. This delicate balance is perturbed when any player in the process is dysfunctional. This week, cases II and III provide examples of this complexity by illustrating the effects of two tumor suppressors that are activated by different stimuli, inhibit different steps of the same canonical signaling pathway, and lead to neuroproliferative disorders when mutated.
Comparisons to the previous case brought a reprise of the hamartoma vs. neoplasia debate during the conference. Conference participants were particularly curious about the ingrowth of the abnormal tissue from a focal area of pigmented leptomeninges at the right brainstem. Participants discussed the invasive nature of this lesion and wondered if this entity might be best considered a low -grade neoplasm. With no consensus reached, the debate rages on (“rage” may be a strong word here.)
References:
- Balne E, Roth DR, Perentes E. Cerebral meningioangiomatosis in a CD-1 mouse: A case report and comparison with humans and dogs. Exp Toxicol Pathol. 2008;60: 247-251.
- Bishop TM, Morrison J, Summers BA, et al. Meningioangiomatosis in young dogs: a case series and literature review. J Vet Intern Med. 2014;18: 522-528.
- Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Elsevier; 2016: 404.
- Corbett MP, Kopec BL, Kent M, et al. Encephalic meningioangiomatosis in a cat. J Vet Diagn Invest. 2022;34(5):889-893.
- Coy S, Rashid R, Stemmer-Rachamimov A, et al. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020;139: 643-665.
- Dantio MC, Dennis AJ, Bergman RL, et al. Surgical treatment of suspected meningioangiomatosis in the thoracolumbar spinal cord. J Am Anim Hosp Assoc. 2020;56(4): e564-01.
- Deb P, Gupta A, Sharma M, et al. Meningioangiomatosis with meningioma: an uncommon association of a rare entity- report of a case and review of the literature. Childs Nerv Syst. 2006;22: 78-83.
- Dono A, Pothiawala AZ, Lewis CT, et al. Molecular alterations in meningioangiomatosis causing epilepsy. J Neuropathol Exp Neurol. 2021;80: 1043-1051.
- Feng R, Hu J, Che X, et al. Diagnosis and surgical treatment of sporadic meningioangiomatosis. Clin Neurol Neurosurg. 2013;115: 1407-1414.
- Frazier K, Liggett A, Hines M 2nd, et al. Mushroom toxicity in a horse with meningioangiomatosis. Vet Hum Toxicol. 2000;42(3): 166-167.
- Ginal PJ, Blanco B, Perez J, et al. Meningioangiomatosis associated with fibrous meningioma in a dog. Vet Rec. 2009;164: 756-758.
- Gonçalves R, Johnston P, Wessmann A, et al. Imaging diagnosis: canine meningioangiomatosis. Vet Radiol Ultrasound. 2010;51(2): 148-151.
- Grabowski MM, Prayson RA. Focal cortical dysplasia in meningioangiomatosis. Clin Neuropathol. 2015;34: 76-82.
- Halper J, Scheithauer BW, Okazaki H, et al. Meningioangiomatosis: a report of six cases with special reference to the occurrence of neurofibrillary tangles. J Neuropathol Exp Neurol. 1986;45(4):426-446.
- Jeffrey M. A neuropathological survey of brains submitted under the Bovine Spongiform Encephalopathy Orders in Scotland. Vet Rec. 1992;131(15): 332-337.
- Li P, Cui G, Wang Y, et al. Multicystic meningioangiomatosis. BMC Neurology. 2014;14:32.
- Lorenzo V, Pumarola M, Munoz A. Meningioangiomatosis in a dog: magnetic resonance imaging and neuropathological studies. J Small Animal Practice. 1998;39: 486-489.
- Makary MS, Kobalka P, Giglio P, et al. Meningioangiomatosis: clinical, imaging and histopathologic characteristics. J Clin Imaging Sci. 2020;10(36): 1-4.
- Marr J, Miranda IC, Miller AD, et al. A review of proliferative vascular disorders of the central nervous system of animals. Vet Pathol. 2021;58 (5): 864-880.
- Morrison H, Sherman LS, Legg J, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15(8):968-980.
- Pumarola M, Martin de las Mulas J, Vilafranca M, et al. Meningioangiomatosis in the brain stem of a dog. J Comp Pathol. 1996;115: 197-201.
- Ribas JL, Carpenter J, Hena H. Comparison of meningioangiomatosis in a man and a dog. Vet Pathol. 1990;27:369-371.
- Sebastianelli M, Mandara MT, Pavone S, et al. Thalamic astrocytic hamartoma and associated meningioangiomatosis in a German shepherd dog. Res Vet Sci. 2013;94: 644-647.
- Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11(6): 471-484.
- Stebbins KE, McGrath JT. Meningio-angiomatosis in a dog. Vet Pathol. 1988; 25: 167-168.
- Tomkinson C, Lu JQ. Meningioangiomatosis: A review of the variable manifestations and complex pathophysiology. J Neurol Sci. 2018;392: 130-136.