Signalment:
Gross Description:
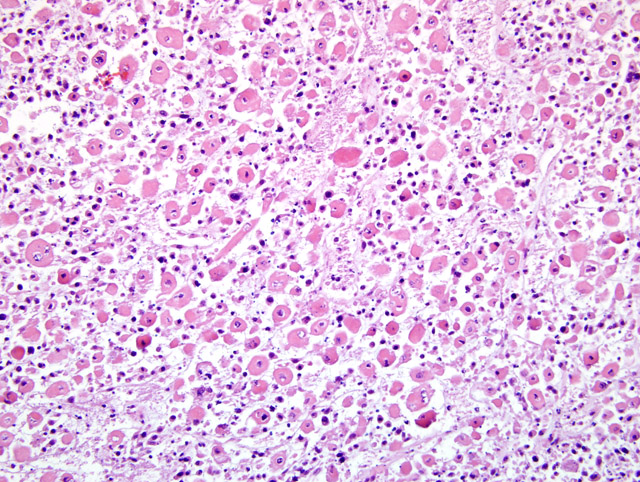
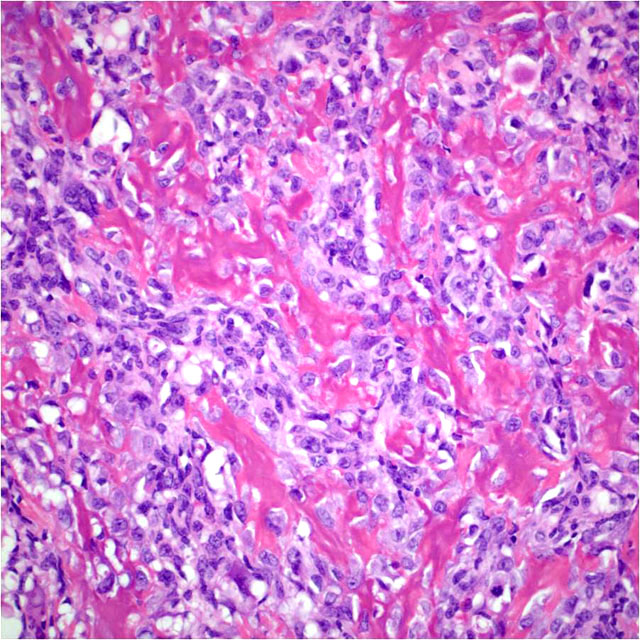
Histopathologic Description:
Within the margins of the tumor adjacent to the lumbar spinal column are multiple fragments of mature cortical bone, necrotic bone, and woven bone. Focally, in association with bone fragments, are small zones of pale basophilic matrix. Along the peripheral margins of the mass, neoplastic cells envelop individualized and atrophic skeletal muscle fibers.
Morphologic Diagnosis:
Lab Results:
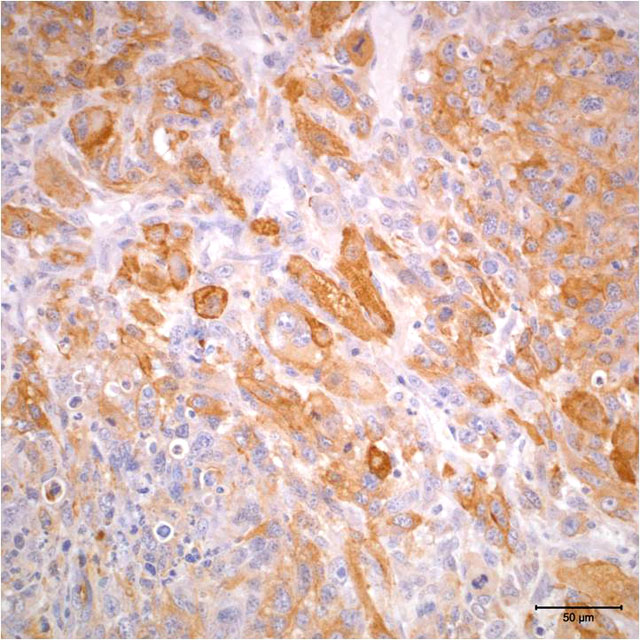
- Vimentin: Diffuse positive cytoplasmic immunoreactivity
- Actin: Multifocal positive cytoplasmic immunoreactivity
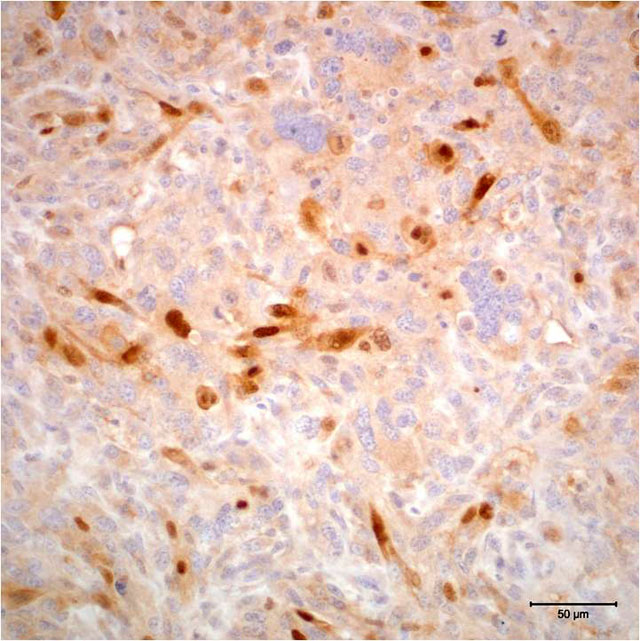
- Myogenin: Multifocal positive nuclear immunoreactivity
- Pancytokeratin: Diffusely negative
Condition:
Contributor Comment:
Most skeletal muscle tumors in animals occur in dogs. Rhabdomyoma and rhabdomyosarcoma have also been reported in cats, cattle, horses, sheep, Sprague-Dawley rats, a Rhesus macaque, and a boa constrictor. In dogs, categorization of rhabdomyosarcomas has included embryonal, botryoid, alveolar, and pleomorphic subtypes, with histologic features of these subtypes in dogs paralleling those described in human cases of rhabdomyosarcoma.(3) The occurrence of laryngeal rhabdomyosarcoma and botryoid rhabdomyosarcoma of the urinary bladder are more commonly described in dogs and may represent distinct clinicopathologic entities in this species.(3)
The degree of expression of immunohistochemical markers is influenced by the degree of differentiation of neoplastic cells, and staining is often heterogeneous within tumors.(3) Immunohistochemical markers that are described for use in the diagnosis of rhabdomyosarcoma include vimentin, desmin, actin, myoglobin, myosin, titin, myogenin, and myoD1 (myogenic determination factor). More traditionally used immunohistochemical markers, such as vimentin, actin, and myoglobin, are not specific for rhabdomyosarcoma; further, they may not be expressed in more poorly differentiated tumors.(2,11) The use of transcriptional factors, specifically myogenin and myoD1, for diagnosis of rhabdomyosarcoma has been shown to demonstrate a greater degree of specificity and sensitivity in human tumors.(2,11) Myogenin and myoD1 are preferentially expressed in the nucleus of differentiating myoblasts and are expressed earlier than desmin, muscle-specific actin, myoglobin, and myosin during skeletal muscle development and differentiation.(2,6)
Immunohistochemical analysis for myogenin expression has previously been applied in a mouse model for methylcholanthrene-induced rhabdomyosarcoma. In this model, both embryonal and pleomorphic rhabdomyosarcomas were induced.(6) Immunohistochemistry for myogenin demonstrated expression of myogenin in both pleomorphic and embryonal variants; embryonal rhabdomyosarcomas exhibited greater myogenin expression than pleomorphic types, and myogenin expression was greatest in neoplastic cells of myoblast-like morphology.(6)
Expression of myogenin and myoD1 in two canine botryoid rhabdomyosarcomas was recently evaluated. (6) In these studies, myogenin and myoD1 expression were found to be useful markers for rhabdomyosarcoma in the dog; histomorphology correlated to labeling for myogenin and myoD1. Specifically, myogenin was predominantly expressed by multinucleated cells that expressed alphasarcomeric actin, resembling myotubes, while myoD1 was expressed by undifferentiated mesenchymal cells.(8)
The case presented herein exhibits histomorphologic features that are most compatible with embryonal rhabdomyosarcoma; however, some regions of the tumor are more suggestive of pleomorphic or alveolar variants, including the presence of numerous large multinucleated neoplastic cells and bundles of spindle cells. Strong and widespread positive immunohistochemical reactivity for vimentin, actin, and myogenin support the diagnosis of rhabdomyosarcoma. The majority of neoplastic cells exhibit strong cytoplasmic immunoreactivity for vimentin and actin. Cells expressing nuclear immunoreactivity for myogenin were frequently small and spindloid; myogenin expression was variably present in multinucleated neoplastic cells.
Interestingly, within the neoplastic mass and distal to the site of pathologic fracture are areas of amorphous eosinophilic matrix resembling osteoid. To our knowledge, osteoid formation within a rhabdomyosarcoma has not previously been described. The origin of this apparent osteoid matrix in this neoplasm is uncertain. No cells within the matrix exhibited immunoreactivity for actin or myogenin, which is in contrast to the majority of the neoplastic population.
Signaling morphogenetic proteins via bone has been implemented in the formation of bone by mesenchymal tissues.(4,10) Bone morphogenetic proteins (BMPs) are cytokines within the transforming growth factor-β (TGF-β) family, and signaling occurs via binding to BMP receptors.(10) While BMP expression has been documented to a variable degree in a number of sarcomas, rhabdomyosarcoma was among a subset of tumors that consistently did not show BMP expression as demonstrated by immunohistochemistry.(15)
The focal zone of osteoid formation may represent a response to bone fracture. Bone formation in muscle, referred to as myositis ossificans or myositis ossificans traumatica, is an infrequent sequelae to bone fracture or muscular trauma with hematoma formation.(1,9) Three types of myositis ossificans traumatica are described; two of these types involve the formation of new bone immediately adjacent to or connected to pre-existing bone. The third type of bone formation occurs within a region of muscle that appears to be separate from underlying bone. Theories for the pathogenesis of this type of bone formation have included escape and proliferation of periosteal osteoblasts, metaplasia of intramuscular connective tissue, or induction of osteogenic precursor cells through BMP signaling.(1) The areas of apparent osteoid formation observed in this rhabdomyosarcoma appear at some distance from the site of bone fracture and thus may be most similar to the third type of myositis ossificans traumatica.
JPC Diagnosis:
Conference Comment:
References:
2. Cessna MH, Zhou J, Perkins SL, Tripp SR, Layfield L, Daines C, Coffin CM: Are myogenin and myoD1 expression specific for rhabdomyosarcoma? Am J Surg Pathol 25:1150-1157, 2001
3. Cooper BJ, Valentine BA: Tumors of muscle. In: Tumors in Domestic Animals, ed. Meuten DJ, pp. 343-357. Iowa State Press, Ames, Iowa, 2002
4. Guo W, Gorlick R, Ladanyi M, Meyers PA, Huvos AG, Bertino JR, Healey JH: Expression of bone morphogenetic proteins and receptors in sarcomas. Clin Orthop 365:175-183, 1999
5. Hendrick MJ, Mahaffey EA, Moore FM, Vos JH, Walder EJ: Histologic Classification of Mesenchymal Tumors of Skin and Soft Tissues of Domestic Animals, 2nd series, vol. 2, pp. 32-33. Armed Forces Institute of Pathology, Washington, DC, 1998
6. Inoue M, Wu H: mmunohistochemical detection of myogenin and p21 in mehtycholanthrene-induced mouse rhabdomyosarcoma. Int J Exp Path 87:445-450, 2006
7. Kempson RL, Fletcher CDM, Evans HL, Hendrickson MR, Sibley RK: Atlas of Tumor Pathology, Tumors of the Soft Tissues, 3rd series, fascile 30, pp. 5. Armed Forces Institute of Pathology, Washington, DC, 1998
8. Kobayashi M, Sakai H, Hirata A, Yonemaru K, Yanai T, Watanabe K, Yamazoe K, Kudo T, Masegi T: Expression of myogenic regulating factors, myogenin and myoD, in two canine botryoid rhabdomyosarcomas. Vet Pathol 41(3):275-277, 2004
9. Kumar V, Abbas A, Fausto N: Cellular adaptations, cell injury, and cell death. In: Robbins and Kotran Pathologic Basis of Disease, ed. Kumar V, Abbas A, Fausto N, 7th ed. p. 11, Elsevier, Philadelphia, PA 2005
10. Nakamura Y, Wakitani S, Saito N, Takaoka K: Expression profiles of BMP-related molecules induced by BMP-2 or -4 in muscle-derived primary culture cells. J Bone Miner Metab 23:426-434, 2005
11. Parham DM, Ellison DA: Rhabdomyosarcoma in adults and children. Arch Path Lab Med 130:1454-1465, 2006
12. Pool RR, Thompson KG: Tumors of joints. In: Tumors in Domestic Animals, ed. Meuten DJ, 4th ed., pp. 241. Blackwell Publishing, Ames, IA, 2002
13. Thompson KG, Pool RR: Tumors of bone. In: Tumors in Domestic Animals, ed. Meuten DJ, 4th ed., pp. 263. Blackwell Publishing, Ames, IA, 2002
14. Vleet JFV, Valentine BA: Muscle and tendon. In: Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 272-277. Elsevier Saunders, Philadelphia, PA, 2007
15. Yoshikawa H, Rettig WJ, Lane JM, Takaoka K, Alderman E, Rup B, Rosen V, Healey JH, Juvos AG, Garin-Chesa P: Immunohistochemical detection of bone morphogenetic proteins in bone and soft-tissue sarcomas. Cancer 74(3):842-847, 1994