Results
AFIP Wednesday Slide Conference - No. 18
January 27, 1999
- Conference Moderator:
LTC David G. Young
US Army Center for Health Promotion and Preventive Medicine
Directorate of Toxicology
Aberdeen Proving Ground, MD 21010-5422
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
Case I - PV98203 (AFIP 2640100)
- Signalment: Three-month-old, female, Maltese dog.
-
- History: This puppy was presented to an emergency
clinic in respiratory distress. It was purchased from a pet shop
a few days before, and the owner wanted only supportive care
until she could return the pup. It was quiet and anxious, with
pale mucous membranes and labored respiratory effort. There was
no history of trauma, nor any oral burns. The pup died after
one hour of oxygen therapy, and neurogenic pulmonary edema was
the tentative diagnosis.
-

- Case 18-1. Note: Though not well visualized here,
the probe extends through the aortic valve, up the aorta, through
a patent ductus arteriosus, and out the pulmonary artery.
-
- Gross Pathology: The clinician performed the postmortem
examination and noted very dark, congested to hemorrhagic lungs,
with abundant edema fluid in all airways. The heart was reportedly
of normal size with no septal or valvular defects, and the veterinarian
stressed that there was no patent ductus arteriosus. The "pluck",
containing the lungs and heart, and liver, kidney and ileum were
received for histopathologic processing.
-
- Laboratory Results: None.
-
- Contributor's Diagnoses and Comments:
- 1. Pulmonary arterial hypertrophy, plexiform, with alveolar
edema.
- 2. Acute bacterial pneumonia.
-
- The pneumonia was judged to be the result of aspiration and
unrelated to the very unusual mural and intimal hypertrophy of
the small pulmonary vessels. There is endothelial hyperplasia,
but no mural fibrinoid necrosis. At prosection, the fixed right
heart seemed hypertrophied as compared to the left, but the heart
base was not further dissected.
-
- This form of plexogenic pulmonary arteriopathy appears to
be scantily represented in veterinary textbooks. In consultation
with the Armed Forces Institute of Pathology, these arterial
lesions were interpreted as consistent with pulmonary hypertension,
either primary or secondary to a left-to-right shunt, such as
a septal defect or patent ductus arteriosus (PDA). The visceral
congestion, pulmonary edema and right ventricular hypertrophy
support that interpretation.
-
- Based on the assessment of the pulmonary histopathology,
the fixed incised heart was retrieved and re-dissected, revealing
a three millimeter patent ductus arteriosus. The probe in the
submitted gross photo has been placed through the lumen from
aorta to pulmonary trunk. In the case of this pup, the patent
ductus must have induced significant secondary pulmonary hypertension
as well as the suggestion of cor pulmonale. This is one extreme
of the spectrum of lesions seen, and not all PDA's are associated
with significant hypertension.
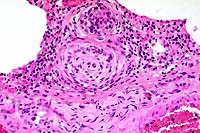 20x
obj
20x
obj
- Case 18-1. Lung. Note intimal and medial hyperplasia
of small arteries which nearly or completely occlude the lumens.
The adjacent interstitium is expanded by lymphocytes, anthracotic
pigment, and cell debris.
-
- AFIP Diagnosis: Lung: Arteriopathy, plexiform, multifocal,
moderate, with diffuse congestion and multifocal alveolar edema,
Maltese, canine.
Note: Some sections may contain minimal acute interstitial
inflammation associated with bacilli.
-
- Conference Note: Pulmonary hypertension induces a
spectrum of vascular lesions in the lung, some of which have
characteristic microscopic features with diagnostic significance.
Although vessel changes may involve the entire arterial tree,
the lesions are most prominent in arterioles and small arteries.
The lesions of the pulmonary vasculature in this Maltese puppy
are characterized by multifocal medial hypertrophy of small to
medium-sized arterioles, loss of the internal elastic lamina,
and extensive hyperplasia of the tunica intima that narrows or
occludes vessel lumens. Occasionally, the hyperplastic endothelium
forms tufts of small, glomerulus-like, capillary channels that
span the lumens of dilated arterioles, resembling a network or
web (hence, plexogenic pulmonary arteriopathy). Multifocally,
there is fibrosis of the alveolar interstitium.
-
- As noted by the contributor, the vascular lesions in this
puppy represent an extreme response to prolonged pulmonary hypertension.
Plexogenic pulmonary arteriopathy is an uncommon finding in domestic
animals. This lesion is an end-stage arterial disease associated
with a poor prognosis, even with correction of the underlying
condition. Pulmonary blood pressure in humans is normally about
one eighth of systemic arterial pressure, and pulmonary hypertension
results when pulmonary vascular pressure reaches one fourth of
systemic levels. Cardiac conditions in animals causing pulmonary
hypertension include anomalies resulting in left-to-right shunts,
usually a ventricular septal defect or PDA. In humans, pulmonary
plexogenic arteriopathy may also occur in cases of idiopathic
primary pulmonary hypertension.
-
- Pulmonary hypertension can also result from any abnormality
that restricts blood flow through the lungs; right ventricular
dilatation with right-sided heart failure often follows, referred
to as cor pulmonale (or pulmonary heart disease). Hypoxia can
be a significant cause of pulmonary hypertension in animals,
and results from any primary disease of the lung, or may occur
as a consequence of reduced atmospheric oxygen, such as that
found at altitudes above 7,000 feet (approximately 2,100 meters).
"High altitude sickness" is most often reported in
cattle. Alveolar hypoxia causes the pulmonary arterioles to constrict.
Hypoxia results in acidosis which also directly induces vasoconstriction
of pulmonary arterioles. Prolonged hypoxia may lead to polycythemia
and increased blood viscosity, further increasing pulmonary blood
pressure.
-
- Participants identified variable numbers of bacilli in the
lung, and some sections contain minimal acute inflammation in
the adjacent pulmonary interstitium. In other sections, no inflammatory
response was found. Participants agreed with the contributor,
and interpreted the bacteria as a probable result of aspiration
shortly prior to death.
-
- Contributor: PATHVET Consultation Services, 3015 Roxanne
Avenue, Long Beach, CA 90808.
-
- References:
- 1. Kolzik L, Schoen FJ: The lung. In: Robbins Pathologic
Basis of Disease, 5th ed., Cotran RS, Kumar V, Robbins SL, Schoen
FJ, eds., pp. 680-682, WB Saunders, Philadelphia, 1994.
- 2. Kuhn C III, Askin FB: Lung and mediastinum. In: Anderson's
Pathology, 9th edition, Kissane JM, ed., vol. 1, pp. 954-958,
CV Mosby, St. Louis, 1990.
- 3. Spencer H: In: Pathology of the Lung (Excluding Pulmonary
Tuberculosis), 3rd edition, vol. 2, pp. 579-615, Pergamon Press,
Oxford, 1979.
- 4. Dungworth DL: The respiratory system. In: Pathology of
Domestic Animals, 4th edition, Jubb KVF, Kennedy PC, Palmer N,
eds., vol. 2, pp. 588-589, Academic Press, San Diego, 1993.
- 5. Robinson WF, Maxie MG: The cardiovascular system. In:
Pathology of Domestic Animals, 4th ed., Jubb KVF, Kennedy PC,
Palmer N, eds., vol. 3, pp. 50-58, 65-66, Academic Press, San
Diego, 1993.
- 6. Jones TC, Hunt RD, King NW: Cardiovascular system. In:
Veterinary Pathology, 6th ed., pp. 983-984, Williams and Wilkins,
Baltimore, 1997.
-
Case II - 96-3954 (AFIP 2648141)
- Signalment: Three to five-month-old, grower pigs.
-
- History: Thirteen out of 45 grower pigs, 3 to 5 months
of age, kept in four different pens, died within two days after
being fed a newly received batch of commercial pig ration supplied
by a small feed manufacturing company. The most obvious symptoms
were dog-sitting postures, posterior paresis, and loud squealing
when disturbed. Death occurred within one to two days of initial
appearance of clinical signs. Two pigs were presented for necropsy
examination.
-
- Gross Pathology: No significant changes were identified
at necropsy. The stomach was moderately filled with fresh residual
food of the same appearance and texture as the ration presented
for examination.
-
- Laboratory Results: Selenium levels as determined
by mass spectrometry in blood (4.9 mg/kg), liver (6.96 mg/kg),
kidney (9.07 mg/kg), residual feed in the stomach (4.89 mg/kg),
and in the feed ration (10.97mg/kg) were high when compared to
maximal permissible levels in blood (0.14-0.19 mg/kg)2,6, liver
(<2 mg/kg)2, kidney (<2mg/kg)2 and feed according to the
level approved for pigs (0.3 mg/kg) by the Food and Drug administration
(FDA) in the USA1,3,8.
-
- Contributor's Diagnosis and Comments: Motoneuronal
necrosis, spinal cord, multifocal, acute, mild.
-
- Hematoxylin and eosin stained transverse and longitudinal
sections of spinal cord of the lumbosacral area are submitted.
There is bilaterally symmetrical degeneration and necrosis of
the motor neurons in the ventral horns of the grey matter. These
changes comprise swelling, chromatolysis, increased eosinophilia,
fading of nuclear membranes, and nuclear pyknosis. In the surrounding
grey matter, there is marked oedema characterized by vacuolation
of the neuropil, dilation of perivascular and perineuronal spaces,
presence of eosinophilic fibrillar to floccular material (probably
protein) in the perivascular spaces, and mild swelling of astrocytes.
Small arterioles in the grey matter reveal mild swelling of endothelial
cells as well as mild fibrinoid change of the walls. A few small
foci of perivascular and interstitial haemorrhage can also be
seen within the grey matter. In the white matter, there is marked
diffuse vacuolar change with mild to moderate axonal swelling.
-
- In contrast to myelomalacia of predominantly the lumbosacral
intumescences described in the literature,3,4,5 in this outbreak,
acute neuronal degeneration and necrosis were noted in the motor
neurons throughout all levels of the spinal cord, from the cervical
to lumbosacral region. There were no other significant histopathological
changes, apart from mild to moderate generalized congestion of
organs, mild to moderate cardiomyopathy, and moderate nephrosis.
Chronic lesions, such as roughness of the haircoat, coronitis
and sloughing of the hooves, have been reported in cases which
survive for longer periods.7 Histopathological changes in such
cases range from multifocal areas of poliomyelomalacia to marked
gliosis with marked gitter cell infiltration, effacement of neurons,
and extensive capillary proliferation.5, 7 .
-
- Supplementation of selenium to commercial rations to prevent
deficiency requires great care due to its potential toxicity
when excessive quantities are added. Maximal levels approved
by the Food and Drug Administration (FDA) of the United States
of America have been set at 0.3 mg/kg.1, 3,6 The history of an
acute posterior paralytic syndrome of sudden onset with a relatively
high morbidity and mortality, toxic levels of selenium in the
feed, liver and kidney, and histopathological changes of acute
neuronal degeneration and necrosis in the spinal cord correspond
with that described for acute selenium intoxication in swine.3,
4,7 Similar clinical signs, macroscopic, and histopathologic
changes have been reproduced in pigs by experimental feeding
of excessive levels of selenium.1,5,9.
-
- Acute selenium toxicosis in pigs should also be distinguished
from poisoning with 6-amininicotinamide (6-AN) which produces
nicotinamide (the amide of niacin, a B complex vitamin) deficiency.
A marked similarity between the clinical signs and histopathological
changes within the spinal cord have been reported with this intoxication
in swine. 6-amininicotinamide is believed to have a toxic effect
on the glial cells of both the central and enteric nervous systems,
and it has been suggested that excessive levels of selenium may
antagonize niacin.
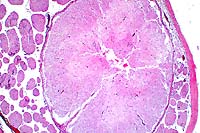 2x
obj
2x
obj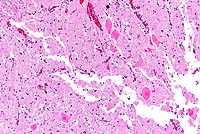 20x
obj
20x
obj
- Case 18-2. Spinal cord. Neuronal degeneration, necrosis,
and loss is responsible for fragmentation & collapse of spinal
cord gray matter. At 20x, neuronal cytoplasm has increased eosinophilia
and nuclei are karyorrhectic.
-
- AFIP Diagnosis: Spinal cord, ventral gray horns: Neuronal
necrosis, bilaterally symmetrical, breed unspecified, porcine.
-
- Conference Note: Excessive supplementation of selenium
in rations is a potential cause of selenium toxicity in animals.
Chronic exposure to environmental sources of selenium is another.
Current concern about selenium bioaccumulation in wetlands from
seleniferous parts of the western United States has stimulated
environmental sampling and studies of waterfowl in these areas.
Selenium contamination of western wetlands results from irrigation
drainage from nearby farmlands. Reduced reproductive success
in waterfowl and fish from selenium contaminated wetlands is
the most sensitive biologic index of toxicity.
-
- The toxicology, clinical signs, and lesions of experimentally
induced selenosis in adult mallard ducks were recently described
in a study that simulated environmental selenium exposure by
varying the concentration of the element added to the ration.
In both subchronically and chronically exposed groups, selenosis
was associated with weight loss, and in several birds, lesions
in the liver and integument. The primary gross lesions in the
subchronic exposure group were emaciation and hepatopathy, while
the most distinctive macroscopic finding in the chronic exposure
group was bilaterally symmetrical alopecia of the crown and neck.
Necrosis of the maxillary beak (maxillary nail) and loss of nails
from the digits also occurred in some chronically exposed birds.
Other lesions that have been attributed to selenium toxicosis
in waterfowl include cardiac lesions, cavitary effusion, pulmonary
congestion or edema, renomegaly, and atrophy of the pancreas,
spleen, and thymus.
-
- Many of the lesions caused by selenosis in waterfowl are
similar to those in mammals, several of which are described by
the contributor. While acute selenium toxicosis may present as
central nervous system disease, chronic selenium toxicosis may
initially manifest as lesions of the integument. Alopecia of
the dorsal midline in pigs and of the mane and tail in horses
is a characteristic feature of selenosis. Experimentally induced
selenosis in cattle causes dyskeratosis of the spinous layer
of hair follicles. Hoof and nail deformity and loss occurs in
cattle, horses, pigs, primates, and humans in subchronic cases
of selenosis. The cutaneous lesions of selenosis are thought
to result from replacement of sulfur in keratin or keratin-associated
proteins by selenium.
-
- Contributor: Pathology Section, P.O. Box 12502, Onderstepoort
0110, South Africa.
-
- References:
- 1. Casteel SW, Osweiler GD, Cook WO: Selenium toxicosis in
swine. J Amer Vet Med Assoc 186:1084-1085, 1985.
- 2. Osweiler GD: Selenium toxicosis. In: Toxicology, pp. 201-203,
Williams & Wilkins Co., Philadelphia, PA, 1996.
- 3. Penrith M-L, Robinson JTR: Acute selenium toxicosis as
a cause of paralysis in pigs. J South African Vet Assoc 66:47-48,
1995.
- 4. Penrith M-L, Robinson JTR: Selenium toxicosis with focal
symmetrical poliomyelomalacia in postweanling pigs in South Africa.
Onderstepoort J Vet Res 63:171-179, 1996.
- 5. Harrison LH, et al.: Paralysis in swine due to focal symmetrical
poliomalacia: Possible selenium toxicosis. Vet Pathol 20:265-273,
1983.
- 6. Stowe HD, et al.: Selenium toxicosis in feeder pigs. J
Amer Vet Med Assoc 201:292-295, 1992.
- 7. Summers BA, Cummings JF, De Lahunta A: Selenium poisoning.
In: Veterinary Neuropathology, 1st edition, pp. 258-261, Mosby
Co., St. Louis, Missouri, 1995.
- 8. Wilson TM, Drake TR: Porcine focal symmetrical poliomyelomalacia.
Canadian J Comp Med 46:218-220, 1982.
- 9. Wilson TM, Scholz RW, Drake TR: Selenium toxicity and
porcine focal symmetrical poliomyelomalacia: Description of a
field outbreak and experimental reproduction. Canadian J Comp
Med 47:412-421, 1983.
- 10. Wilson TM, Cramer PG, Owen RL: Porcine focal symmetrical
poliomalacia: Test for an interaction between dietary selenium
and niacin. Canadian J Vet Res 53:454-461, 1989.
- 11. O'Toole D, Raisbeck MF: Experimentally induced selenosis
of adult mallard ducks: Clinical signs, lesions, and toxicology.
Vet Pathol 34:330-340, 1997.
-
Case III - 98-3345 (AFIP 2639842)
-
- Signalment: A nine-month-old, aborted, equine fetus.
-
- History: Spontaneous abortion occurred during the
ninth month of gestation, with no significant clinical signs
in the mare.
-
- Gross Pathology: The fetus was well-preserved. There
was congestion and interlobular edema of the lungs, and pleural
effusion. There was congestion of the liver and excess peritoneal
fluid.
-
- Laboratory Results: Bacterial cultures were negative.
Equine herpesvirus type I was demonstrated in the thymus, lungs
and liver by the fluorescent antibody technique.
-
- Contributor's Diagnosis and Comments: Multifocal necrotizing
bronchopneumonia with eosinophilic intranuclear inclusion bodies.
Etiology : Equine herpesvirus type I.
This is a good example of the lung lesions caused by equine herpesvirus.
There is diffuse congestion with interlobular and subpleural
edema. There are multiple foci of necrosis and hemorrhage in
the pulmonary parenchyma. Cells surrounding the necrotic foci
contain small eosinophilic intranuclear inclusion bodies. Necrosis
of bronchial and bronchiolar epithelium is also present, with
cellular debris in the lumens, and intranuclear inclusions are
present in cells near the necrotic areas. Focal necrosis and
intranuclear inclusions were also present in the liver, spleen
and thymus.
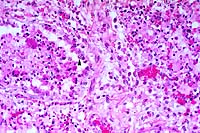 20x
obj
20x
obj
- Case 18-3. Lung. There is diffuse bronchiolar epithelial
necrosis with scattered eosinophilic intranuclear inclusions
(arrowhead), and several syncytial cells in various stages of
degeneration. The bronchial lumen is filled with cell debris
and the pulmonary interstitium is expanded by edema, a mixed
cellular infiltrate, and cellular debris.
-
- AFIP Diagnosis: Lung: Pneumonia, broncho-interstitial,
necrotizing, acute, diffuse, severe, with syncytial cells, eosinophilic
intranuclear inclusion bodies, edema, hemorrhage, and fibrin,
breed unspecified, equine, etiology consistent with equine herpesvirus
type I.
-
- Conference Note: Equine herpesvirus type 1 (EHV-1) is currently
one of three a-herpesviruses known to produce disease in horses.
EHV-1 is associated with abortion, neonatal death, respiratory
disease, and neurologic dysfunction, and has been referred to
as equine abortion virus. The other two equine a-herpesviruses
include EHV-3, the cause of equine coital exanthema, and EHV-4,
which is primarily associated with respiratory disease (equine
viral rhinopneumonitis) in young horses, but may also cause abortion.
Thus, both EHV-1 and EHV-4 may cause respiratory disease and
abortion; however, EHV-4 more frequently causes respiratory disease,
and EHV-1 is the important cause of single and multiple abortions
in mares. Furthermore, EHV-1 is the only equine herpesvirus which
causes neurologic disease. EHV-2 and EHV-5 are g-herpesviruses
as determined by DNA fragment analysis. Both have been associated
with upper respiratory disease, though the pathogenic importance
of EHV-2 is questionable since antibodies are commonly found
in healthy and diseased horses.
-
- EHV-1 is a potentially serious cause of reproductive disease
in mares, and both sporadic and epizootic late-term abortions
and stillbirths may occur. Typically, abortion is observed between
the eighth and eleventh months of gestation. The fetus is usually
aborted quickly without premonitory signs in the mare, and the
fetus is fresh and well-preserved. The fetus is usually alive
when abortion begins, and becomes asphyxiated during delivery.
Gross examination of the abortus often reveals meconium staining
of the amnion and fetus due to fetal diarrhea initiated by asphyxia.
The presence of meconium and squames in bronchioles and alveoli
in the examined sections of lung in this case suggests an attempt
to breath by the fetus, and is consistent with previous observations
of EHV-1 induced abortion. The most consistent gross lesions
in the fetus are severe pulmonary edema and pleural effusion.
Grossly and microscopically, necrosis may be present in the lungs,
liver, spleen, and lymph nodes, and inclusions often occur adjacent
to necrotic areas, especially in the liver and lungs. The placenta
is usually unaffected.
-
- EHV-1 may also manifest as severe respiratory disease in
newborn foals infected late in gestation, and as central nervous
system dysfunction in adult horses. Most foals infected with
EHV-1 are born weak and eventually die, though some reports suggest
beneficial affects of early acyclovir treatment in foals surviving
infection. Sporadic cases of myeloencephalitis in adult horses
caused by EHV-1 infection have been identified since the early
1970's. The disease results in sudden onset of ataxia that may
rapidly progress to paralysis. Adult horses acquire the virus
through exposure to an aborted fetus. Histologic lesions may
occur in the brain, spinal cord, meninges and ganglia, characterized
by nonsuppurative vasculitis with medial and endothelial necrosis.
The vascular lesions may result in poliomalacia or leukomalacia.
The virus does not appear to be neurotropic, and the character
of the vascular changes suggests an immune complex type disease.
-
- Contributor: Department. of Pathology and Microbiology,
Faculty of Veterinary Medicine, Univer. of Montreal, C.P. 5000,
St.Hyacinthe, Quebec, Canada J2S 7C6.
-
- References:
- 1. Jones TC, Hunt RD, King NW: Diseases caused by viruses.
In: Veterinary Pathology, 6th ed., pp. 230-232, Williams and
Wilkins, Baltimore, 1997.
- 2. Murray MJ, et al.: Neonatal equine herpesvirus type 1
infection on a thoroughbred breeding farm. J Vet Intern Med 12:36-41,
1998.
- 3. Telford EA, et al: Equine herpesviruses 2 and 5 are g
herpesviruses. Virology 195:492-499, 1993.
- 4. Rooney JR, Robertson J: Respiratory system and female
reproductive system. In: Equine Pathology, pp. 38-39, 246-248,
Iowa State Univ. Press, 1996.
- 5. Kennedy PC, Miller RB: The female genital system. In:
Pathology of Domestic Animals, Jubb, Kennedy, Palmer, eds., 4th
ed., vol. 3, pp. 436-438, Academic Press, San Diego, CA, 1993.
-
Case IV - 192R, 195R, 192L (AFIP 2656744)
-
- Signalment: Nine to ten-week-old, Crl:CD (SD) BR rat.
-
- History: This rat was part of a group of positive
controls dosed with 0.8% theophylline for 36 days.
-
- Gross Pathology: None.
-
- Laboratory Results: No significant findings.
-
- Contributor's Diagnoses and Comments:
- 1. Testis: Moderate to severe, segmental testicular degeneration
with giant cell formation, tubular dilation, sperm stasis and
spermatocyte necrosis.
- 2. Moderate to severe, multifocal vasculitis (not all sections).
- 3. Rete testis: Moderate to severe sperm stasis with spermatocele.
-
- Theophylline (1,3-dimethylxanthine) is closely related to
caffeine. Theophylline has been used therapeutically to treat
respiratory disorders due to its ability to relax smooth muscle.
When given in large quantities, theophylline has been reported
to cause testicular degeneration.
-
- The testicular changes include formation of multinucleated
giant cells (derived from coalescence of spermatids), retention
of spermatozoa with sperm stasis, loss of germ cells (spermatogonia
and spermatocytes) and tubular dilation. Sperm stasis in the
rete testis with spermatocele formation likely contributed to
the tubular dilation. Tubular dilation has also been proposed
to be caused by theophylline suppression of seminiferous tubular
contraction.
-
- In some sections, there is a vasculitis/perivasculitis of
a testicular blood vessel. Theo-phylline has been reported to
cause vasculitis in rats, primarily affecting the mesenteric
vessels. However, vascular inflammation can be found in many
organs of theophylline-exposed rats.
-
- The associated epididymal sections are largely devoid of
spermatozoa. In some sections, there is a mild hyperplasia of
the epididymis at the junction of the caput and cauda. The thickened
epididymal epithelium forms infoldings. One animal also had spermatocele
formation with progression to spermatic granuloma in the epididymis.
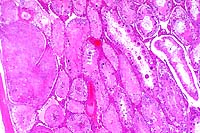 4x
obj
4x
obj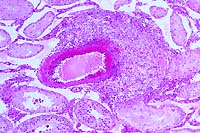
- Case 18-4. The left photo illustrates degeneration
and loss of germ cells with marked dilation of a tubule by dense
aggregates of spermatozoa and secretions (spermatocele). Other
tubules are filled with ill defined debris including multinucleate
giant cells which are barely visible. The image on the right
demonstrates vasculitis and perivasculitis with fibrinoid change.
-
- AFIP Diagnoses:
- 1. Testis: Degeneration and necrosis, germ cells, with numerous
multinucleate spermatitic giant cells, and multifocal spermatoceles,
Crl:CD (SD) BR rat, rodent.
2. Testis: Vasculitis, chronic-active, focal, moderate, with
fibrinoid change and mild multifocal pericapsular lymphoplasmacytic
perivasculitis and interstitial edema.
3. Epididymis: Sperm granuloma, focal.
Some sections contain peri-epididymal edema.
-
- Conference Note: Theophylline, a methylxanthine closely
related to caffeine and theobromine, occurs in small amounts
in coffee and tea. As noted by the contributor, the drug relaxes
smooth muscle of the pulmonary vasculature and bronchial airways,
and also acts as a cardiac stimulant and diuretic. In humans,
theophylline has been used in the treatment of asthma, emphysema,
and certain cardiovascular conditions. Due to concerns about
potential drug-induced neoplasia in humans subjected to prolonged
theophylline therapy, oral feeding studies were conducted in
which rats were given high doses of theophylline, theobromine,
and caffeine. While these methylxanthines did not induce neoplastic
or preneoplastic changes in treated animals, several other important
toxicopathologic manifestations were identified, including histopathologic
changes in the testes similar to those present in this rat. The
testicular changes were most pronounced in caffeine and theobromine
treated rats, and less severe in animals treated with theophylline.
-
- Marked testicular changes have also been described in rabbits
fed theobromine. Lesions were characterized as degeneration and
necrosis of germ cells, formation of multinucleated spermatids
and spermatocytes, and hemorrhage, congestion, and interstitial
edema. Additionally, myocardial degeneration and necrosis, severe
hemorrhage and premature involution of the thymus were found.
In contrast, the characteristic clinicopathologic finding of
iatrogenically induced theobromine toxicity in dogs is right
atrial cardiomyopathy. Accidental methylxanthine poisoning has
been reported in animals, and is usually a result of ingestion
of excessive chocolate (theobromine) or over-the-counter stimulants
by pets. Accidental theobromine toxicity usually manifests as
central nervous system signs which begin as restlessness, hyperactivity,
and urinary incontinence, and may progress to twitching, spastic
contraction of muscles, and convulsions.
-
- Differentiating testicular lesions induced by theophylline
administration from degenerative changes resulting from other
causes was a topic of discussion at the conference. Testicular
maturation varies among species and strains. In rabbits, there
is also significant variation among individuals of the same strain
and age. Spermatitic giant cells (multinucleated spermatids/giant
cells) have been interpreted as a degenerative change, but also
have been reported in normal rats, rabbits, mice, dogs, pigs,
and men. Microscopic degenerative lesions of the testes have
been reported in ten to twelve-week-old Crl:CD/BR rats used as
untreated controls in oral and inhalation toxicity studies. Varying
degrees of seminiferous tubule degeneration with giant cell formation
was described. While the mean incidence of testicular degenerative
changes in control rats used in oral studies was low (2.5%),
some seminiferous tubules had advanced stages of degeneration,
including tubules lined by only Sertoli cells. Further, inanition
is a clinical condition that may affect germ cell maturation.
Theophylline is known to cause reduced food consumption in experimental
laboratory animal studies. Thus, there may be several possible
causes of germ cell degeneration and giant cell formation in
the testis, including hypoxia due to vascular changes, sperm
stasis as a result of smooth muscle relaxation, inanition, and
age, strain, or individual animal variation. In experimental
studies involving the testes, all potential causes of degenerative
changes should be considered.
-
- Contributor: Pfizer Central Research, Eastern Point
Road, Groton, CT 06340.
-
- References:
- 1. Friedman L, et al.: Testicular atrophy and impaired spermatogenesis
in rats fed high levels of the methylxanthines caffeine, theobromine,
or theophylline. J Environ Pathol Toxicol 2:687-706, 1979.
- 2. Lindamood C, et al.: Studies on the short-term toxicity
of theophylline in rats and mice. Fundam Appl Toxicol 10:477-489,
1988.
- 3. Morrissey RE, Collins JJ, Lamb JC, Manus AG and Gulati
DK: Reproductive effects of theophylline in mice and rats. Fundam
Appl Toxicol 10:525-536, 1988.
- 4. Weinberger MA, et al.: Testicular atrophy and impaired
spermatogenesis in rats fed high levels of the methylxanthines
caffeine, theobromine, or theophylline. J Environ Pathol Toxicol
1:669-688, 1978.
- 5. Yuan YD, Kennedy AH and Ochoa R: Testicular toxicity of
theophylline in rats. Toxicol Pathol 22:655, 1994.
- 6. Soffietti MG, et al.: Toxic effects of theobromine on
mature and immature male rabbits. J Comp Pathol 100:47-58, 1989.
- 7. Lee KP, Frame SR, Sykes GP, Valentine R: Testicular degeneration
and spermatid retention in young male rats. Toxicol Pathol 21:292-302,
1993.
- 8. Frame SR, Hurtt ME, Green JW: Testicular maturation in
prepubertal New Zealand white rabbits. Vet Pathol 31:541-545,
1994.
- 9. Morton D, et al.: Spermatid giant cells, tubular hypospermatogenesis,
spermatogonial swelling, cytoplasmic vacuoles, and tubular dilatation
in the testes of normal rabbits. Vet Pathol 23:176-183, 1986.
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
- Return to WSC Case Menu

 20x
obj
20x
obj

 20x
obj
20x
obj
 2x
obj
2x
obj 20x
obj
20x
obj
 20x
obj
20x
obj
 4x
obj
4x
obj