Results
AFIP Wednesday Slide Conference - No. 15
January 6, 1999
- Conference Moderator:
Dr. Jerrold Ward
National Cancer Institute
NCI-FCRDC
Fairview 201, P.O. Box B
Frederick, MD 21702-1201
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I - 98-005744 or 98-005745 (AFIP 2640348)
-
- Signalment:
5744: Female, 21-week-old, 129 x C57Bl x EIIa-cre transgenic
mouse.
5745: Male, 16-week-old, 129 x C57Bl x EIIa-cre transgenic mouse.
-
- History: Heterozygous mice (+/-) for the acid a-glucosidase
(GAA) gene were crossed to EIIa-cre transgenic mice for Cre-mediated
deletion of the neo gene and exon 6 of the GAA gene. As mice
aged, they had decreased body weight gain and developed clinical
signs of muscle weakness. Later, older mice died.
-
- Gross Pathology: None.
-
- Laboratory Results: None.
-
- Contributor's Diagnoses and Comments:
- 1. Heart and skeletal muscle: Degeneration, vacuolar, severe.
- 2. Heart and skeletal muscle: Regeneration, moderate.
Etiology: Abnormal storage of glycogen in cardiac myocytes
and skeletal muscle. Condition: Glycogen Storage Disease Type
II.
-
- The periodic acid-Schiff (PAS) reaction shows that many vacuoles
are PAS positive and diastase sensitive (longer diastase digestion
times may have to be used). No evidence of heart failure has
been seen as yet. Mice will be used as models for gene therapy.
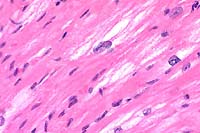 40x
obj
40x
obj
- Case 15-1. Heart. Moderate numbers of cardiac myocytes
contain large vacuoles.
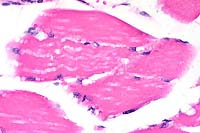 40x
obj
40x
obj
- Case 15-1. Skeletal muscle. Muscle fibers have cytoplasmic
vacuolation.
-
- AFIP Diagnosis: Heart and skeletal muscle: Degeneration,
vacuolar, diffuse, moderate, with multifocal regenerative changes,
129 x C57Bl x EIIa-cre transgenic mouse, rodent.
Conference Note: Glycogen is present in variable amounts
in all cells where it serves as a readily available source of
energy for cellular activities. In humans, several genetic disorders
have been identified that result from a metabolic defect in the
synthesis or degradation of glycogen. The best understood category
of such derangements of glycogen metabolism are the glycogen
storage diseases (glycogenoses). The glycogenoses result from
a hereditary deficiency of one of the enzymes involved in the
synthesis, transport, or degradation of glycogen, leading to
accumulation of glycogen within cells in abnormal quantities
or with an abnormal structure.
-
- More than a dozen glycogenoses have been identified in humans,
but the disorders may be broadly classified into three major
subgroups based on pathophysiology and tissue locations of abnormally
accumulated glycogen: hepatic forms, myopathic forms, and the
glycogenoses associated with deficiency of a-glucosidase or lack
of branching enzymes. The liver is an important organ in glycogen
metabolism. Hepatic forms of glycogen storage disease occur when
hepatic enzyme deficiencies involved in glycogen synthesis and/or
degradation lead to abnormal storage of glycogen in the liver
and hypoglycemia. In contrast to the liver, striated muscle primarily
consumes glycogen as a source of energy. Myopathic forms of the
disease result from a deficiency of enzymes involved in the glycolytic
pathway that lead to impaired energy production and storage of
glycogen in muscles.
-
- The final major subgroup of the glycogenoses, those associated
with either a deficiency of a-1,4-glucosidase (acid maltase)
or lack of a branching enzyme, lead to glycogen storage in many
organs and often early death, although milder adult forms of
disease may occur. Type II glycogenosis (Pompe disease) results
from deficiency of lysosomal acid maltase leading to abnormal
storage of glycogen within lysosomes in all organs. In contrast
to type II glycogenosis, the other forms are cytosolic and do
not primarily involve lysosomes. In humans, the infantile form
of Pompe disease results in cardiomegaly and rapidly progressive
heart failure. In the adult form, affected individuals may die
from pulmonary failure secondary to diaphragmatic weakness, though
some survive into the seventh decade of life.
-
- Type II glycogen storage disorders have been reported in
several animal species including the Lapland dog (infantile form),
Shorthorn cattle (both infantile and late-onset forms), Brahman
cattle (late onset form only) and Japanese quail (late onset
form only). The disease has also been reported in a cat and Corriedale
sheep. While laboratory rodents have not been used extensively
as a source of models for spontaneously occurring glycogen storage
disorders, recent techniques in gene targeting and disruption
in mice have increased the numbers and types of animals available
for study. Slight alterations in the genetic background of mice
of similar strains appear to contribute to distinct clinical
phenotypes present at different ages. Mice with homozygous mutation
for type II glycogen storage disorder present with several clinical
and pathologic features of both the infantile and adult forms
of the disease. The mice submitted by the contributor in this
case have only been slightly genetically altered from homozygous
mice.
-
- Contributor: National Cancer Institute, NCI-FCRDC,
Fairview 201, PO Box B, Frederick, MD 21702-1201.
-
- References:
- 1. Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L,
LaMarca M, King C, Ward JM, Sauer B, Plotz P: Targeted disruption
of the acid a-glucosidase gene in mice causes an illness with
the critical features of both infantile and adult human glycogen
storage disease type II. J Biol Chem 273:19086-19092, 1998.
- 2. Becker, et al.: The African origin of the common mutation
in African American patients with glycogen storage disease type
II. Am J Hum Genet 62:991-994, 1998.
- 3. Jolly RD, Walkley SU: Lysosomal storage diseases of animals:
An essay in comparative pathology. Vet Pathol 34:527-548, 1997.
- 4. Jones TC, Hunt RD, King NW: Intracellular and extracellular
depositions; degenerations. In: Veterinary Pathology, 6th ed.,
pp. 241-245, Williams and Wilkins, Philadelphia, 1997.
- 5. Cotran RS, Kumar V, Collins T: Genetic disorders. In:
Robbins Pathologic Basis of Disease, 6th ed., pp. 160-163, WB
Saunders, Philadelphia, 1999.
-
-
- Case II - No Label (AFIP 2643751)
Signalment: 18-month-old, male, Crl:CDâ(SD)Br rat
(Rattus norvegicus).
-
- History: This rat was in a control group in a two-year
carcinogenicity study and was euthanized in moribund condition
after approximately 16 months of treatment.
Gross Pathology: A spherical mass approximately 15 mm
in diameter was found in the right seminal vesicle at necropsy.
-
- Laboratory Results: None.
-
- Contributor's Diagnosis and Comments: Carcinoma, scirrhous,
seminal vesicle.
- This neoplasm was one of two neoplasms that appeared at necropsy
to arise from the seminal vesicles in this study of approximately
350 rats. These neoplasms may invade surrounding organs, making
it difficult to determine if the origin is the seminal vesicle
or the anterior prostate (coagulating gland). In this case, the
gross findings suggested that the carcinoma arose from the seminal
vesicles. This rat also had a large pituitary neoplasm that was
thought to be the cause of the rat's clinical condition and sacrifice.
-
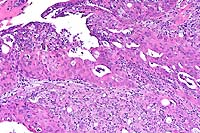 10x
obj
10x
obj
- Case 15-2. Seminal vesicle. The submucosa is heavily
infiltrated by pleomorphic epithelial cells. Both mucosal papillary
fronds and submucosa have an abundant inflammatory (neutrophilic)
infiltrate.
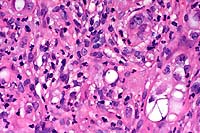 40x
obj
40x
obj
- Case 15-2. Seminal vesicle. Pleomorphic tumor cells,
which are admixed with neutrophils in the submucosa may contain
multiple nuclei or variably sized vacuoles which displace the
nucleus peripherally (signet ring cells).
-
- AFIP Diagnosis: Seminal vesicle: Adenocarcinoma, Crl:CDâ(SD)Br
rat, rodent.
-
- Conference Note: Replacing most of the normal glandular
parenchyma and compressing the adjacent coagulating gland is
an unencapsulated, densely cellular, expansile, lobular neoplasm
composed of polygonal cells arranged in nests, cords, papillary
fronds, and glandular structures, separated and supported by
a moderate to coarse fibrovascular stroma. Frequently, the nests
and fronds of neoplastic cells are surrounded by abundant, immature,
fibrous connective tissue (desmoplasia). The glandular ducts
sometimes contain small amounts of amorphous, brightly eosinophilic
secretory material and/or degenerate neutrophils. Neoplastic
cells have variably distinct cell borders with small to moderate
amounts of basophilic to amphophilic cytoplasm, and irregularly
round to oval nuclei that are vesicular or have stippled chromatin,
and one to three magenta nucleoli. The mitotic rate is high,
and there are focally extensive areas of necrosis.
-
- As noted by the contributor, distinguishing between adenocarcinomas
of the coagulating gland and seminal vesicle may be difficult,
especially when the tumor is large and involves adjacent organs.
Determining the site of origin grossly is often troublesome,
especially when tumors are located in the proximal part of the
seminal vesicle adjacent to the dorsolateral prostate. Histologically,
both tumors feature a glandular pattern with prominent stroma.
-
- Most conference participants favored seminal vesicle as the
primary site of origin, because many sections contain areas in
which there appears to be transition from normal glandular epithelium
of the seminal vesicle to the neoplasm, although this finding
is not present in all histologic sections. Other histologic features
of the tumor consistent with seminal vesicle origin include elongated
fronds of basophilic epithelium, small amounts of brightly eosinophilic
secretory product within glandular structures, and the scirrhous
reaction. The coagulating gland contains smaller papillary projections
and forms a pale, eosinophilic secretory substance compared to
the seminal vesicle; the epithelium is lightly eosinophilic rather
than basophilic. Tumors of the coagulating gland tend to be smaller
and less schirrhous than those of the seminal vesicle.
-
- While rats have a high incidence of spontaneous prostatic
tumors, spontaneous neoplasms of the seminal vesicles are rare,
with fewer than ten cases reported. Neoplasia of the seminal
vesicles is rare in humans and domestic animals as well. Experimentally,
neoplasia of the seminal vesicle can be induced in rats by exposure
with several carcinogenic agents, including N-methylnitrosourea
and irradiation. Because of the scarcity of reports of spontaneous
seminal vesicle neoplasia in rats, little is known about the
biological behavior of these tumors. A recent case report of
a seminal vesicle adenocarcinoma in a Fischer 344 rat described
a pleomorphic tumor with numerous mitoses but no observed metastases.
-
- Contributor: Abbott Laboratories, Department of Pathology,
D-469 AP13A, 100 Abbott Park Road, Abbott Park, IL 60064-3500.
-
- References:
- 1. Boorman GA, Elwell MR: Male accessory sex glands, penis,
and scrotum. In: Pathology of the Fischer Rat, Boorman G, et
al., eds., pp. 424-425, Academic Press, San Diego, CA, 1990.
- 2. Shirai T, Takahashi S, Tamano S: Preneoplasia and neoplasia
of the rat male genital tract. In: Pathology of Neoplasia and
Preneoplasia in Rodents, Bannasch P, Gossner W, eds., pp. 146-154,
Schattauer, Stuttgart, GE, 1997.
- 3. Shoda T, et al.: A spontaneous seminal vesicle adenocarcinoma
in an aged F344 rat. Toxicol Pathol 26:448-451, 1998.
- 4. Bosland MC, et al.: Proliferative lesions of the prostate
and other accessory sex glands in male rats, URG-4. In: Standardized
System of Nomenclature and Diagnostic Criteria Guides for Toxicological
Pathology, pp. 1-10, Society for Toxicological Pathology, American
Registry of Pathology, and the Armed Forces Institute of Pathology,
Washington DC, 1998.
-
-
- Case III - 97-000835, 97-106095, or 97-000839 (AFIP 2639847)
Signalment: 19-month-old, female mice, 129 x C57BL CYP1A2
-/- (knockout).
-
- History: Mice are usually clinically normal.
Gross Pathology: At the glandular stomach along the junction
with the forestomach there are round to elongated, raised plaques
which measure 5-15 mm.
-
- Laboratory Results: None.
-
- Contributor's Diagnoses and Comments:
- 1. Gastric fundic hyperplasia and metaplasia, focal, severe.
- 2. Gastritis, focal, moderate.
- 3. Herniation of epithelial cysts, submucosa and tunica muscularis,
moderate (in slide #97-106095).
-
- Slide #98-000835 has areas in which many epithelial cells
contain eosinophilic, hyaline cytoplasm; fewer similar cells
are seen in slide #97-106095.
-
- The pathogenesis of the hyperplasia and metaplasia is not
known. We have found similar lesions in wild type mice (CYP1A2
+/+) on 129 and 129 x C57Bl backgrounds. The other regions of
the stomach are usually normal. Similar and other types of gastric
hyperplasia have been report in other knockouts and transgenic
mice (see references).
-
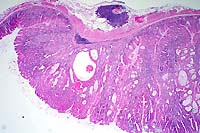 2x
obj
2x
obj
- Case 15-3. Glandular Stomach. Hyperplastic zone is
3-4x as thick as adjacent normal mucosa. Some crypts are cystic.
There ae both submucosal and subserosal lymphoid follicles.
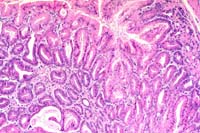 10x
obj
10x
obj
- Case 15-3. Glandular stomach. Mucosal glands are occasionally
cystic and diffusely hyperplastic, but uniform and well oriented
to their basement membrane.
-
- AFIP Diagnosis: Stomach, glandular: Hyperplasia, adenomatous,
focally extensive, with mild multifocal lymphoplasmacytic gastritis,
129 x C57BL CYP1A2 -/- (knockout) mouse, rodent.
-
- Conference Note: A focally extensive area of the gastric
glandular mucosa at the junction with the squamous stomach is
thickened five to ten times normal. This plaque-like thickening
is characterized by long, arborizing papillary fronds of hyperplastic
mucosa usually lined by a single layer of tall columnar epithelial
cells. Scattered throughout the mucosa are variably-sized, dilated
or cystic glands partially filled eosinophilic proteinaceous
material occasionally admixed with mineralized debris. A mixture
of parietal cell, chief cell, and mucous neck cell hyperplasia
is present, and occasionally the epithelial cells pile-up several
layers thick. Some sections contain gastric glands that have
herniated through the tunica muscularis into the tunica serosa.
Scattered within the submucosa and serosal adipose tissue there
are nodular aggregates of lymphocytes and macrophages.
-
- The contributor of this case (the conference moderator) has
recently observed large, broad-based, papillary to polypoid lesions
of the glandular stomach in these knock-out, transgenic mice.
The lesions in the affected strains are consistently observed
at the junction of the squamous and glandular stomach, begin
as small, plaque-like thickenings of the glandular mucosa, and
occur in mice eighteen months of age or older. The squamous portion
of the stomach is usually unaffected. There is an 80% incidence
rate in affected mice strains, and lesions increase in size as
mice age.
-
- In mice that are homozygous deficient for the aryl-hydrocarbon
receptor (AHR), a wide variety of phenotypic alterations occur
in major organ systems. Gastric mucosal hyperplasia commonly
occurs in the pyloric stomach of AHR-deficient mice, and with
age the lesions progress to gastric polyps. The aryl-hydrocarbon
receptor is a ligand-activated transcription factor and is thought
to be intimately involved in cellular proliferation, normal development,
and physiologic homeostasis in several body systems. In the MT100
mouse, transforming growth factor-a (TGF-a) is overexpressed
in the gastric glandular mucosa, and severe adenomatous hyperplasia
of the glandular mucosa results characterized by proliferation
of mucin-secreting cells and atrophy of parietal and zymogen
secreting cells. The MT100 mouse strain serves as a model for
the human condition known as Menetrier's disease, and the microscopic
features and expression pattern of TGF-a in the gastric mucosa
are similar in both. Chronic hypertrophic gastritis in dogs is
an idiopathic condition which most often affects the body of
the canine stomach, has similar microscopic features to Menetrier's
disease, and may or may not cause clinical signs.
- Conference participants briefly discussed whether this lesion
represented a neoplastic or hyperplastic/metaplastic process,
most agreeing that the lesion is not neoplastic. The mixture
of proliferating mucous neck cells, parietal cells, and chief
cells is not consistent with the clonal expansion of a single
cell line expected with a neoplastic process.
-
- Contributor: National Cancer Institute, NCI-FCRDC,
Fairview 201, PO Box B, Frederick, MD 21702-1201.
-
- References:
- 1. Pineau T, et al.: Neonatal lethality associated with respiratory
distress in mice lacking cytochrome P450 CYP1A2. Proc Nat Acad
Sci USA 92:5134-5138, 1995.
- 2. Takagi H, et al.: Histochemical analysis of hyperplastic
stomach of TGF-a transgenic mice. Dig Dis Sci 42:91-98, 1997.
- 3. Bockman DE, Sharp R, Merlino G. Regulation of terminal
differentiation of zymogenic cells by transforming growth factor
alpha in transgenic mice. Gastroenterology 108:447-454, 1995.
- 4. Sharp R, et al.: Transforming growth factor alpha disrupts
the normal program of cellular differentiation in the gastric
mucosa of transgenic mice. Development 121:149-161, 1995.
5. Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ:
Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol
34:605-614, 1997.
- 6. Barker IK, van Dreummel AA, Palmer N: The alimentary system.
In: Pathology of Domestic Animals, Jubb KVF, Kennedy PC, Palmer
N, eds., 4th ed., volume 2, page 62, Academic Press, San Diego,
CA, 1993.
-
-
- Case IV - C90-0013 (AFIP 2641930)
-
- Signalment: Tissue from multiple, ten-week-old, female,
CBA/CaJ mice.
-
- History: This group of mice was thymectomized at six
weeks of age and irradiated three weeks later. Seven to ten days
following irradiation, several animals died unexpectedly.
-
- Gross Pathology: Gross examination revealed shrunken
spleens and irregular yellow and white foci scattered throughout
the liver parenchyma.
-
- Laboratory Results:
- 1. Bacterial culture: Negative
2. Serology: Sentinel mice from the involved room, which had
been previously negative for antibody to murine viral pathogens,
were serologically positive for mouse hepatitis virus.
- Contributor's Diagnosis and Comments: Liver: Multifocal necrotizing
hepatitis with syncytial giant cells.
Etiology: Mouse hepatitis virus (MHV).
-
- Death is generally not associated with MHV infection except
when the infection occurs in immunocompromised mice. Syncytial
cells are commonly seen in murine coronavirus infection. A diagnosis
of MHV is based on the liver lesions with syncytial giant cells
and the serological analysis of the room sentinel mice.
-
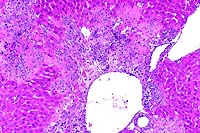 10x
obj
10x
obj
- Case 15-4. Liver. Severe focal to coalescing necrosis
is associated with inflammatory cell infiltration and syncytial
giant cells.
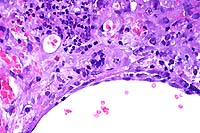 40x
obj
40x
obj
- Case 15-4. Liver. Areas of hepatocellular necrosis
are replaced by necrotic cellular debris, hemorrhage, neutrophils
and occasional clusters of nuclei (hepatocyte syncytium). An
adjacent hepatic vein contains endothelial cell hypertrophy and
hyperplasia.
-
- AFIP Diagnosis: Liver: Necrosis, random, multifocal
to coalescing, with syncytia and mild neutrophilic inflammation,
CBA/CaJ mouse, rodent.
-
- Conference Note: Mouse hepatitis virus (MHV) is a
murine coronavirus with several antigenic strains characterized
by variability in virulence and tissue tropism. MHV is broadly
classified into enterotropic and respiratory tropic groups, depending
upon the primary target organ. Enterotropic strains tend to infect
only the intestinal mucosa, and the virus is usually not disseminated
to other tissues. Respiratory strains, on the other hand, are
considered polytropic. The virus replicates in the nasal mucosa
and spreads via lymphatics and the vascular system to the lungs
where replication in pulmonary vascular endothelium occurs. Secondary
viremia develops with spread to multiple organs, including the
liver and brain. Most MHV strains are tropic for the respiratory
system, and hepatitis is most often associated with respiratory
strains of MHV.
-
- Various factors influence both the susceptibility and clinical
outcome of MHV infection, including virus strain, route of infection,
diet, concurrent infections, age and immune status of mice. In
immunocompromised mice, the consequences of MHV infection depend
upon whether animals are infected with the respiratory or enterotropic
coronaviral strain. Infection with respiratory MHV in athymic
or SCID mice results in progressive, fatal, multisystemic disease
with severe necrotizing lesions in the nasal mucosa, liver, brain,
bone, lymphoid tissue, bone marrow, and other tissues. Compensatory
splenic extramedullary hematopoiesis often results in splenomegaly.
Virulent strains of respiratory MHV produce rapid death in immunodeficient
mice, while infection with less virulent strains causes chronic
wasting. Enterotropic MHV may cause chronic enteric disease in
immunocompromised mice, but animals usually do not manifest clinically
apparent disease.
-
- Conference participants identified prominent syncytia at
the periphery of necrotic foci within the liver; infrequent endothelial
syncytia are present in some sections. Participants briefly discussed
several potential causes for random hepatic necrosis in the mouse,
including Tyzzer's disease (Clostridium piliforme), salmonellosis,
and mousepox (ectromelia virus). Tyzzer's disease and salmonellosis
do not have syncytial cells as a characteristic microscopic finding.
If present in sections, salmonella organisms may be demonstrated
by tissue Gram stains as short, Gram-negative, bacilli. Necrosis
of Peyer's patches frequently occurs with enteric salmonellosis.
Silver stains, such as the Warthin-Starry, best demonstrate the
bacteria in Tyzzer's disease. They appear as filamentous bacilli
within hepatocytes at the periphery of necrotic areas. In the
liver lesions of mousepox, intracytoplasmic inclusions are evident
in hepatocytes at the periphery of necrotic foci, and syncytial
cells are not present. Cutaneous lesions, splenic necrosis, and
necrosis of lymph nodes and Peyer's patches are often present
in cases of mousepox.
-
- Contributor: Comparative Medicine Division, St. Jude's
Children Research Hospital, 332 North Lauderdale Street, Memphis,
TN 38105.
-
- References:
- 1. Percy DH, Barthold SW: Mouse. In: Pathology of Laboratory
Rodents and Rabbits, Barthold SW, ed., pp. 20-23, Iowa State
University Press, Ames, Iowa, 1993.
- 2. Barthold SW: Mouse hepatitis virus infection. In: Monographs
on Pathology of Laboratory Animals: Digestive System, Jones TC,
Popp JA, Mohr U, eds., pp. 179-184, Springer-Verlag, Berlin,
Germany, 1985.
- 3. Mickelsen SL, Greenlee PG: Diagnostic exercise: Vascular
endothelial lesions in athymic nu/nu mice. Lab Anim Sci 48:92-94,
1998.
-
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
- Return to WSC Case Menu
 40x
obj
40x
obj
 40x
obj
40x
obj
 10x
obj
10x
obj
 40x
obj
40x
obj
 2x
obj
2x
obj
 10x
obj
10x
obj
 10x
obj
10x
obj
 40x
obj
40x
obj