Results
AFIP Wednesday Slide Conference - No. 12
December 2, 1998
- Conference Moderator: Dr. Bruce H. Williams,
Diplomate, ACVP
Department of Telepathology
Armed Forces Institute of Pathology
Washington, DC 20307
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I - 98-8397 (AFIP 2648048)
-
- Signalment: One-year-old, spayed female, Siberian
Husky, canine.
-
- History: This dog had a history of a stick lodged
in the mouth six months prior to biopsy. The dog was presented
with approximately five, variably-sized, raised, ulcerated, ovoid
masses in the oral cavity measuring 1 to 5 centimeters in diameter.
A single "granulomatous" mass was excised from the
frenulum of the tongue for histopathology.
-
- Gross Pathology: A formalin-fixed, 1.5 x 3.5 centimeter,
raised, ulcerated lingual mass was submitted for histopathology.
Contributor's Diagnosis and Comments: Tongue, frenulum
(per contributor): Severe, chronic, multifocal to coalescing
eosinophilic granuloma with collagen degeneration and rare intralesional
bacteria.
-
- The mass is consistent with a canine eosinophilic granuloma,
a rare syndrome characterized by oral or cutaneous lesions. The
most common clinical presentation is focal disease of the oral
cavity. Although there is a marked breed predilection for the
Siberian Husky, typically in males less than three years of age,
lingual eosinophilic granulomas have been reported in a Bull
Mastiff and a mixed-breed dog. Plaque-like lesions typically
develop on the lateral or ventral surface of the tongue in Siberian
Huskies. Palatal lesions have been reported in several breeds.
While the exact cause is not known, the striking breed predilection
suggests possible hereditary factors. Proposed mechanisms include
trauma, insect bites and foreign bodies. In this case, the dog
had a history of a stick foreign body lodged in the mouth six
months prior to biopsy. Complete excision is not recommended
as deformities may result, and the lesions respond readily to
glucocorticoids.
 2x
obj
2x
obj
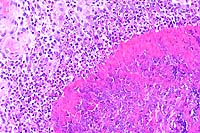
- Case 12-1. Oral mucosa with extensive ulceration (except
for lower right corner) & multifocal areas of liquefactive
necrosis surrounded by both suppurative and granulomatous inflammation.
 20x
obj
20x
obj
- Case 12-1. Oral mucosa. Necrotic foci are centered
on an eosinophilic coagulum composed of necrotic cells and denatured
protein which forms picket fense-like radial projections (Splendore-Hoeppli
phenomenon). This material is surrounded by myriad eosinophils,
fewer neutrophils, and more distant accumulations of epithelioid
macrophages.
-
- AFIP Diagnosis: Oral mucosa (per contributor): Stomatitis,
proliferative, eosinophilic and granulomatous, focally extensive,
severe, with collagen degeneration and ulceration, Siberian Husky,
canine.
-
- Conference Note: This pedunculated to polypoid lesion
is covered by an extensively ulcerated stratified squamous nonkeratinizing
epithelium. Within the mass, there are multifocal to coalescing
nodular aggregates of eosinophils and macrophages that palisade
around degenerate, fragmented, and sometimes hyalinized, bundles
of collagen. Lymphocytes and plasma cells surround the eosinophilic
granulomatous nodules, and immature fibrous connective tissue
forms the peripheral boundaries of these inflammatory foci.
-
- As noted by the contributor, canine eosinophilic granuloma
may have various causes but a common histopathologic presentation.
Lesions are grossly and histologically similar to those in the
cat. The disease most commonly presents as single to multiple
ulcerated lesions in the oral cavity, often on the lateral or
ventral surfaces of the tongue or on the soft palate. The cutaneous
form occurs less frequently and is characterized by multiple
papules, nodules, and plaques primarily on the ventral abdomen,
flanks and prepuce. Rarely, solitary lesions in the external
ear canal may occur.
-
- The etiology of canine eosinophilic granuloma is unknown,
but hereditary and/or hypersensitivity mechanisms are proposed
based upon breed predilection and response to glucocorticoid
therapy. While the syndrome has been reported in several breeds,
male Siberian huskies are classically described as the most predisposed,
especially to the oral form of the disease. Recently, two separate
reports from the United States and Europe describe oral eosinophilic
granulomas in several Cavalier King Charles spaniels; affected
animals were relatively young (four years or less), and most
were male. Thus, Cavalier King Charles spaniels may also be predisposed
to the oral form of canine eosinophilic granuloma.
Contributor: University of Illinois - Laboratories of
Veterinary Diagnosis, 2001 South Lincoln, Urbana, IL 61802.
-
- References:
- 1. Madewell BR, Stannard AA, Pulley LT, Nelson VG: Oral eosinophilic
granuloma in Siberian husky dogs. J Amer Vet Med Assoc 177:701-703,
1980.
- 2. Potter KA, Tucker RD, Carpenter JL: Oral eosinophilic
granuloma of Siberian huskies. J Amer Anim Hosp Assoc 16:595-600,
1980.
- 3. Walsh KM: Oral eosinophilic granuloma in two dogs. J Amer
Vet Med Assoc 183:323-324, 1983.
- 4. Scott DW: Cutaneous eosinophilic granulomas with collagen
degeneration in the dog. J Amer Anim Hosp Assoc 19:529-532, 1983.
- 5. Gross TL, Ihrke PJ, Walder EJ: Nodular and diffuse diseases
of the dermis with prominent eosinophils or plasma cells. In:
Veterinary Dermatopathology, pp. 218-220, Mosby-Year Book, St.
Louis, MO, Year Book, 1992.
- 6. Bredal BP, et al.: Oral eosinophilic granuloma in three
Cavalier King Charles spaniels. J Small Anim Pract 37:499-504,
1996.
- 7. Yager JA, Scott DW: The skin and appendages. In: Pathology
of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th
ed., volume 1, pp. 699-702, Academic Press, San Diego, CA, 1993.
-
- Case II - 93W 9960-4 or 93W 9896-3 (AFIP 2642052)
-
- Signalment: One-year-old, female, Siberian polecat
x black-footed ferret hybrid (Mustela eversmanni x M. nigripes).
-
- History: Hybrid ferrets consumed laboratory mice experimentally
infected with Yersinia pestis to determine susceptibility of
ferrets to oral plague. This work was associated with the recovery
program for the endangered black-foo-ted ferret. Most hybrid
ferrets were febrile (temperatures >40 C) and anorectic by
two days post consumption of a single in-fected mouse. They became
depressed, and after a clinical course of 3 to 7 days, became
moribund and were euth-anized.
-
- Gross Pathology: This ferret was in excellent body
condition (856 g). The mandibular and retropharyngeal lymph nodes
were great-ly enlarged, hemorrhagic, and necrotic. Associated
soft tissues were slightly edematous. The lungs were edematous
and mottled. There was increased clear fluid in the thoracic
cavity which contained a few fibrin strands. The gastro-intestinal
tract was empty except for black tarry feces in the distal colon.
Laboratory Results: Impression smears of lung, liver,
spleen, and lymph nodes were positive for Y. pestis by direct
fluorescent anti-body tests. Yersinia pestis was cultured from
pooled tissues and lymph nodes. No antibodies against Y. pestis
were detected in serum.
-
- Contributor's Diagnosis and Comments: Retropharyngeal
lymph node: Lymphadenitis, necrotizing and suppurative, diffuse,
severe, with hemorrhage, edema, vasculitis, thrombosis, and numerous
coccobacilli (Yersinia pestis), Siberian polecat x black-footed
ferret.
-
- The typical lesions of plague in susceptible species are
necrotizing and hemorrhagic lymphadenitis (bubo forma-tion),
septicemia, and pulmonary edema and hemorrhage associated with
vascular damage. The lesions in cervical lymph nodes reflect
entry of the bacteria through the oropharyngeal mucosa to the
regional nodes with subsequent proliferation of bacteria and
septicemia. If exposure to plague is by flea bite, buboes develop
at regional lymph nodes, typically inguinal and axillary. Gram-negative
coccobacilli are present in large numbers in the nodes and ves-sel
lumina. Disseminated intravascular coagulation and endotoxic
shock occur in highly susceptible individuals.
-
- Carnivores, with the exception of Felidae, have been thought
to be relatively resistant to developing clinical plagu-e; most
rodents are highly susceptible. However, a single case in a black-footed
ferret demonstrated that at least some members of the genus Mustela
are susceptible to fatal infection. Subsequent studies demonstrated
that hy-brid ferrets (used as surrogates for black-footed ferrets)
are highly susceptible to plague by oral or parenteral routes
of exposure. This is important in the recovery program for the
black-footed ferret, because sylvatic plague is com-mon throughout
much of the west and is particularly common in prairie dogs,
the primary prey of black-footed ferrets.
 2x
obj
2x
obj
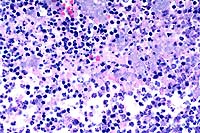
- Case 12-2. Lymph node. Extensive necrosis and inflammation
with focal areas of hemorrhage.
 40x
obj
40x
obj
- Case 12-2. Lymph node. Multifocally within zones suppuration
and necrosis, are multiple large colonies of bacteria.
 40x
obj, Brown & Brehn
40x
obj, Brown & Brehn
- Case 12-2. Perinodal fat. Brown & Brehn staining
reveals that bacterial colonies are composed of short reddish-blue
(Gram negative) rods.
- AFIP Diagnosis: Lymph node: Lymphadenitis, necrotizing,
suppurative, diffuse, severe, with numerous bacilli, Siberian
polecat x black-footed ferret (Mustela eversmanni x M. nigripes),
mustelid.
-
- Note: Necrosis, suppurative inflammation and hemorrhage
are present within the surrounding perinodal tissues in some
sections.
-
- Conference Note: Yersinia pestis is a nonmotile, non-spore
forming, facultative anaerobic, gram-negative, bipolar coccobacillus
of the family Enterobacteriaceae and is the cause of the human
disease known as "plague". The organism exists on every
continent except Australia, and is found most often in cool,
semiarid climates. While the organism is sensitive to dessication,
it can survive several weeks to months in organic material such
as infected carcasses.
-
- Wild rodents, such as prairie dogs and ground squirrels,
serve as reservoirs for infection of humans and domestic animals.
The wild rodent population serves as the source of infection
through chronic bacteremia; transmission of Y. pestis to susceptible
hosts, including humans, occurs through flea bites, by contact
of the organism with mucous membranes (ingestion) or broken skin,
or by inhalation of droplets from animals with pneumonic plague.
-
- Flea bites are the most common mode of transmission among
wild rodents and to humans. After fleas ingest blood from an
infected host, Y. pestis multiples in the insect's gut and produces
coagulase. Coagulase causes coagulation of ingested blood at
subsequent feedings, obstructing the flea gastrointestinal tract.
Obstruction of the gut causes regurgitation of bacteria into
the wound of the next mammalian host.
-
- Once the organism enters the mammalian host, the initial
pathogenesis of infection depends upon whether infection occurs
through a flea bite or through the mucous membranes or broken
skin. After a flea bite, the organisms are phagocytized by neutrophils
and macrophages. In neutrophils, the organism is destroyed, but
in mononuclear cells the organism not only survives but multiplies.
Multiplication of Y. pestis within host cells depends upon the
presence of a virulence plasmid or pathogenicity island called
Yop. Yop encodes a type III secretion apparatus and numerous
proteins that disrupt normal host cell signal transduction pathways,
including a serine-threonine kinase and a protein tyrosine phosphatase3.
Infected macrophages travel to regional lymph nodes where the
organism ruptures the infected phagocytic cells, replicates,
and eventually causes lymphadenitis, lymphoid necrosis, and abscess
formation (buboes). Initial replication in macrophages results
in the production of a capsular envelope, rendering the organism
resistant to further phagocytosis. Y. pestis makes a plasmid-encoded
protease that activates plasminogen and cleaves complement C3
at a specific site. This secreted protease is essential for spread
of the bacteria from the local site of inoculation into the bloodstream;
mutant bacteria lacking this protease are much less virulent.
-
- In contrast to flea inoculation, organisms that are ingested
or inhaled from contaminated tissue or fluids by susceptible
hosts have already acquired the phagocytic-resistant capsule
from the previous host's macrophages, and thus the organism spreads
more rapidly resulting in a shorter incubation time. The oral
route of transmission is most common in cats, ferrets, and other
carnivores predatory on rodents. The presence of a bacterial
capsule at the time of exposure, coupled with higher numbers
of organisms received from ingested infected rodents, are likely
the key reasons which make susceptible mammalian carnivores vulnerable
to oral routes of transmission, but refractory to percutaneous
inoculation by fleas.
-
- In fatal cases of plague, bacteria overwhelm lymph nodes,
and organisms become distributed throughout the host via the
lymphatic channels or bloodstream. During bacteremia organisms
may become disseminated to the eye, liver, kidney, spleen, brain,
and lung. Yersinia pestis contains endotoxins that may result
in edema, septic shock, and disseminated intravascular coagulation.
-
- Contributor: Wyoming State Veterinary Laboratory,
1174 Snowy Range Road, Laramie, Wyoming 82070.
-
- References:
- 1. Williams ES, Thorne ET, Quan TJ, Anderson SL: Experimental
infection of domestic ferrets (Mus-tela putorius furo) and Siberian
polecats (Mustela eversmanni) with Yersinia pestis. J Wildl Dis
27:441-445, 1991.
- 2. Williams, ES, Mills K, Kwiatkowski DR, Thorne ET, Boerger-Fields
A: Plague in a black-footed fer-ret (Mustela nigripes). J Wildl
Dis 30:581-585, 1994.
3. Sameulson J: Infectious diseases. In: Robbins Pathologic Basis
of Disease, Cotran RS, Kumar V, Collins T, eds., 6th ed., pp.
387-388 and 356, WB Saunders, Philadelphia, PA, 1999.
- 4. Macy DW: Plague. In: Infectious Diseases of the Dog and
Cat, Greene CE, ed., 2nd ed., pp. 295-300, 1998.
-
- Case III - 98-1252 (AFIP 2644339)
-
- Signalment: Seven-year-old, spayed female, Domestic
Longhair, feline.
-
- History: A bulging iris was noted in the right eye
of this cat at the time of its yearly vaccination. The owner
had noted the change approximately one month prior, and stated
that the change had progressed. There was no apparent discomfort
to the animal.

- Case 12-3. Eye. As described below.
- Gross Pathology: An irregularly shaped, pale tan mass
measuring approximately 0.75 cm in diameter was present within
the globe and extended caudally from the iris.
-
- Contributor's Diagnosis and Comments: Eye: Iridociliary
adenocarcinoma.
- A partially encapsulated, highly cellular mass consisting
of cuboidal to polygonal cells arranged in loose cords, packets,
and occasional rosettes is adherent to the posterior aspect of
the iris and to the ciliary body. The neoplasm infiltrates the
base of the iris and extends into the filtration angle. Irregularly
shaped, dilated channels are present in some areas, and the mass
is supported by a fine fibrovascular stroma. Cells within the
mass have large, round to oval, occasionally indented nuclei,
1-2 nucleoli, finely stippled chromatin, small to moderate amount
of foamy, eosinophilic cytoplasm, and variably-distinct to indistinct
cell margins. A few cells have large, irregularly shaped nuclei,
and the mitotic rate varies from 0-3 per high-powered field.
Some scleral vessels adjacent to the neoplasm contain thrombi
and seemingly have "infiltrates" of cells (may not
be visible in all sections); the cells are dissimilar to those
within the neoplasm and may, in fact, represent a reaction to
thrombosis or other negative vascular events.
 2x
obj
2x
obj
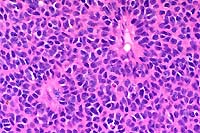
- Case 12-3. Eye. A monomorphic mass replaces the iris.
 40x
obj
40x
obj
- Case 12-3. Ocular tumor. Sheets of pleomorphic polygonal
cells occasionally palisade around a central lumen (pseudorosette
formation).
-
- AFIP Diagnosis: Eye: Ciliary body adenocarcinoma,
Domestic Longhair, feline.
- Note: some sections contain minimal cataractous change.
-
- Conference Note: This neoplasm was studied in consultation
with the Department of Ophthalmic Pathology. Conference participants
agreed with the contributor's diagnosis. An expansile, infiltrative,
densely cellular neoplasm has effaced the ciliary body and is
composed of polygonal cells arranged in packets, nests, tubular
structures, and pseudorosettes, supported by a fine fibrovascular
stroma. Neoplastic cells have significant atypia, with occasional
bizarre cells. The mitotic rate is high. Scattered within the
tumor are entrapped melanophages which contain moderate amounts
of brown-black granular to globular pigment.
-
- Immunohistochemical studies performed at the AFIP demonstrate
that the tumor is multifocally positive for both keratin and
vimentin, and negative for S-100 protein. The PAS reaction demonstrates
that neoplastic cells align along a PAS-positive basement membrane,
supporting the diagnosis of ciliary body adenocarcinoma.
-
- Primary tumors of the globe are occasionally encountered
in dogs and cats. Melanocytic neoplasms are most frequently reported,
while those originating from the ciliary body epithelium are
the second most commonly encountered. Ciliary body neoplasms
arise from mature ciliary body epithelium, which is of neuroectodermal
origin. Medulloblastomas and retinoblastomas are primary ocular
tumors that arise from embryonic neuroectoderm.
-
- Tumors of the ciliary body may be pigmented or nonpigmented,
depending upon whether the neoplastic cell population arises
from the inner nonpigmented or outer pigmented layer of the ciliary
epithelium; nonpigmented tumors are more common than pigmented
tumors. Tumors of nonpigmented epithelium tend to produce thick
basement membranes, while tumors of the pigmented epithelium
tend to form solid darkly pigmented masses. Ciliary body adenomas
are more common than adenocarcinomas in both dogs and cats. Adenomas
tend to grow endophytically, while adenocarcinomas are more likely
to invade adjacent tissues. Metastasis is rare in dogs and cats
with ciliary body adenocarcinoma, but may occur in advanced stages
of disease. In humans, ciliary body tumors are rare, and adenomas
occur more frequently than adenocarcinomas. There are few convincing
reports of metastatic disease.
Differential diagnosis discussed by participants included melanoma
and metastatic carcinoma. The Department of Ophthalmic Pathology
considers the PAS reaction the most important laboratory procedure
for differentiation of ciliary body adenocarcinoma from melanoma.
Ciliary body adenocarcinomas are characterized by PAS-positive
basement membranes, while melanomas are not.
-
- Contributor: Department of Pathology, College of Veterinary
Medicine, The University of Tennessee, PO Box 1071, Knoxville,
TN 37901.
-
- References:
- 1. Wilcock BP: The eye. In: Pathology of Domestic Animals,
Jubb KVF, Kennedy PC, Palmer N, eds., 4th ed., volume 1, pp.
519-520, Academic Press, San Diego, CA, 1993.
- 2. Peiffer Jr RL: Ciliary body epithelial tumours in the
dog and cat: A report of thirteen cases. J Small Anim Pract 24:347-370,
1983.
- 3. Dubielzig RR: Ocular neoplasia in small animals. In: Small
Animal Ophthalmology, Vet Clin N Amer 20(3):837-848, 1990.
- 4. Bellhorn RW: Ciliary adenocarcinoma in the dog. J Amer
Vet Med Assoc 159:1124-1128, 1971.
- 5. Gionfriddo JR, et al.: Ocular manifestations of a metastatic
pulmonary adenocarcinoma in a cat. J Amer Vet Med Assoc 197:372-374,
1990.
- 6. Shields JA, et al.: Acquired neoplasms of the nonpigmented
ciliary epithelium. Ophthalmology 103:2007-2016, 1996.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame # 7926, 9395, 9451, 9456, 16852-53, 16928.
-
-
- Case IV - Unlabeled 8x10 EM photo (print) (AFIP 2648170)
-
- Signalment: Male, Fischer 344 rat.
-
- History: This control rat was given a cyclodextrin
vehicle.
-
- Histopathologic Findings:
-
- Kidney, hematoxylin and eosin stained sections.
- Multifocally, proximal renal tubules have swollen and vacuolated
epithelial cells. Affected epithelial cells contain variably-sized,
intracytoplasmic, eosinophilic, granular deposits and low numbers
of eosinophilic, hyaline droplets and crystals.
-
- Contributor's Diagnosis and Comments: Kidney, proximal
tubules: Renal tubular degeneration with intralysosomal amorphous
material and crystals (alpha 2m globulin).
 40x
obj
40x
obj
- Case 12-4. Kidney. The cytoplasm of many proximal
tubules is rarified, somewhat foamy, and often contains globular
to to polygon shaped eosinophilic inclusions.

- Transmission Electron Micrograph, 1540X. Kidney, proximal
tubules. The electron micrograph illustrates portions of four
proximal tubules lined by tall cuboidal epithelial cells with
a brush border, oval nuclei with dispersed chromatin and 1-2
small nucleoli, and abundant mitochondria, many arrayed perpendicularly
to the basement membrane. The epithelial cells are swollen, vacuolated,
and have numerous, intracytoplasmic osmiophilic amorphous deposits
(secondary lysosomal contents) surrounded by an electron-lucent
space and a single membrane (fused lysosomes) and large, rectangular,
rhomboid or irregular to needle-like crystalline intralysosomal
deposits (alpha 2m globulin). Interspersed between the proximal
tubules (lower left) are small spindle cells with oval nuclei
(mesangial cells).
-
- This case is an example of two lesions, both lysosomal. The
crystalline deposits are due to deposition of alpha 2m globulin,
while the fused or coalesced secondary lysosomes distended with
amorphous material are consistent with a lysosomal storage disorder.
-
- Alpha 2m globulin deposits may be globular, rectangular,
rhomboid or irregular in shape. Alpha 2m globulin is produced
in large quantities in the liver of male rats, and accumulates
as hyaline droplets in the renal tubular epithelium. Numerous
chemicals can disrupt the metabolism of alpha 2m globulin, resulting
in an exacerbation of protein deposition and more rapid development
of nephropathy due to tubular degeneration and necrosis.
-
- The other lysosomal alterations are consistent with a lysosomal
storage disorder, in this case due to cyclodextrin administration.
Cyclodextrins are widely used in oral, topical and parenteral
pharmaceutical preparations to increase solubility and form stable
complexes that result in enhanced drug delivery. Toxicity with
these compounds varies with the specific type of cyclodextrin.
Renal lesions, specifically proximal tubular degeneration and
necrosis, are associated with methylated cyclodextrins, particularly
TM-beta-cyclodextrins. Although the exact mechanism for the tubular
lesion is unknown, these compounds disrupt phagosomal-lysosomal
fusion. Although acicular microcrystals have been reported with
some cyclodextrins, their origin and significance have not been
determined. In the case presented here, crystals were consistent
with alpha 2m globulin.
AFIP Diagnosis: Kidney, proximal convoluted tubular epithelium:
Degeneration, multifocal, moderate to severe, with cytoplasmic
vacuolation, variably electron-dense acicular crystals, and electron-dense
rhomboidal and globular bodies, Fischer 344 rat, rodent.
-
- Conference participants generally agreed with the following
description of the submitted electron micrograph:
-
- Kidney, proximal convoluted tubule: There are portions
of at least three tubular structures, each lined by contiguous
cuboidal to rectangular cells aligned along a prominent basement
membrane. Along the luminal border of these cells are lush microvilli.
The cells have irregularly oval nuclei which contain abundant
euchromatin and peripherally clumped heterochromatin. One nucleus
has two small nucleoli. The cytoplasm contains abundant, closely
packed, elongate mitochondria that are often arranged perpendicularly
to the basement membrane. There is a moderate amount of rough
endoplasmic reticulum within the cytosol. Multifocally near the
cell apices there are few pinocytotic vesicles. Interposed between
adjacent tubular basement membranes are a few small cells with
scant cytoplasm and oval to angular nuclei (fibroblasts or other
interstitial cells).
-
- Multifocally within the cytoplasm of the tubular epithelial
cells there is an accumulation of numerous irregularly shaped,
variably-sized, electron-lucent vacuoles that often coalesce
(enlarged lysosomes). Superimposed within these vacuolated areas
are numerous smaller, intensely electron-dense, variably-sized
granules. Within most tubular epithelial cells in the vacuolated
areas there are few electron-dense crystals that vary from thin
spicules with sharply pointed ends, to large hexagonal or rhomboidal
crystalline structures. The enlarged lysosomes containing the
previously described material displace mitochondria and nuclei
peripherally.
-
- Conference Note: This case was reviewed in consultation
with Dr. David Fritz, consultant to the Department of Veterinary
Pathology for ultrastructural studies.
-
- Case 12-4. Electron micrographs
- Many of the tubular epithelial cells in the central tubule
at the center of the photo are swollen and expanded more laterally
than apically due to the vacuolated inclusions. Residual cellular
organelles are peripheralized and compartmentalized. The compartmentalized
mitochondria have lost proper orientation and are no longer aligned
perpendicular to the basement membrane. In the epithelial cell
in the center of the photo, the lateral cell boundaries are markedly
widened, and the nucleus is compressed and flattened against
the cell base (see cell labeled "4" at AFIP website).
Because these ultrastructural changes probably alter normal cellular
function, the morphologic diagnosis of "cellular degeneration"
is appropriate.
-
- Extensive intracytoplasmic vacuolation is present in the
tubular epithelium. Determining the nature of these vacuoles
is difficult due to the low magnification of the electron micrograph,
but there is evidence that the vacuoles represent enlarged and
giant lysosomes. First, of the various organelles that can become
dilated in renal tubular epithelial cells, only the lysosome
regularly contains material of varying size, shape, and density.
The material within many of these vacuolated structures appears
to be multiple lysosomes within one unit membrane. In one or
two vacuoles, the individual lysosomal membranes disappear, forming
one large vacuole (see center bottom tubular epithelial cell
in photo, or refer to AFIP website with cell labeled "1").
Second, several vacuoles contain homogenous, medium electron-dense
material suggestive of lysosomal contents (see cell labeled "2"
on AFIP website). Normally, lysosomal contents are very electron-dense
when tissue is fixed in 1% glutaraldehyde; however, the contributor
does not mention method of tissue fixation in this case, and
the preservation of lysosomal material may have been altered
by an alternative fixative.
The globular to rhomboidal, electron-dense, intracytoplasmic
bodies present in the tubular epithelial cells are consistent
with the alpha 2m globulin hyaline inclusions seen in rat hyaline
droplet nephropathy. However, the intracytoplasmic acicular (needle-like)
crystals observed in this case are not characteristic of rat
hyaline nephropathy, but rather are more consistent with the
microcrystals observed in cyclodextrin-induced nephrosis in the
male rat. The renal toxicity of cyclodextrins is manifested ultrastructurally
as increased vacuoles within the apical cytoplasm of the proximal
tubular epithelial cells, with the eventual formation of giant
lysosomes, and the presence of acicular microcrystals within
the lysosomal matrix. Cyclodextrins are known to form complexes
with several cellular compounds, including lipids, cholesterol,
and lipoproteins. The acicular crystals may represent cyclodextrin
complexed to alpha 2m globulin in renal tubular epithelium.
-
- Contributor: Lilly Research Laboratories, PO Box 708,
Greenfield, IN 46140.
-
- References:
- 1. Alden CL, Frith CH: Urinary system. In: Handbook of Toxicology,
Haschek WM, Rousseaux CG, eds., pp. 316-388, Academic Press Inc.,
San Diego, CA, 1991.
- 2. Thompson DO: Cyclodextrins-enabling excipients: Their
present and future use in pharmaceuticals. In: Critical Reviews
in Therapeutic Drug Carrier Systems, Bruck SD ed., 14(1):1-104,
Begell House Inc., New York, 1997.
- 3. Frank DW, Gray JE, Weaver RN: Cyclodextrin nephrosis in
the rat. Am J Comp Path 83:367-382, 1976.
- 4. Haschek WM, Rousseaux CG: The kidney. In: Fundementals
of Toxicologic Pathology, pp. 173-177, Academic Press, San Diego,
CA, 1998.
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
Return to WSC Case Menu
 2x
obj
2x
obj
 20x
obj
20x
obj
 2x
obj
2x
obj
 40x
obj
40x
obj
 40x
obj, Brown & Brehn
40x
obj, Brown & Brehn

 2x
obj
2x
obj
 40x
obj
40x
obj
 40x
obj
40x
obj
