Results
AFIP Wednesday Slide Conference - No. 30
27 May 1998
Conference Moderator:
LTC Thomas P. Lipscomb, Diplomate, ACVP
Division of Veterinary Pathology
Armed Forces Institute of Pathology
Washington, D.C. 20306
Return to WSC Case Menu.
Case I - S-51-96 (AFIP 2593311)
Signalment: Free-ranging, male, pink river dolphin (Inia
geoffrensis).
History: This animal was captured in the course of a
radio-tagging field study conducted in Lago Mamiraua, Brazil.
Gross Pathology: A heavily vascularized, multinodular,
pedunculated mass was attached to the right eye. It was firmly
adhered to, and appeared to arise from, the conjunctiva of the
dorsal palpebrum as well as the medial and lateral canthus.
Contributor's Diagnosis and Comments: Conjunctiva: Conjunctivitis,
proliferative, chronic-active to granulomatous, diffuse, marked,
with mucosal hyperplasia and intralesional fungal elements compatible
with Rhinosporidium seeberi.
The polyploid conjunctival lesions seen in this dolphin are "classic"
examples of an infection with Rhinosporidium seeberi. Although
a number of different blocks were used for glass slide recuts,
all slides demonstrate the developmental stages of R. seeberi.
These are readily identified by specific histologic features.
These are:
- 1. Trophocyte (juvenile sporangia)
- - averages 10-100 microns
- has a 2-3 micron unicellular wall
- each has a central nucleus with prominent nucleolus
- granular to flocculent cytoplasm
- no mature endospores
-
- 2. Intermediate sporangia
- - are larger than trophocytes
- these lack a nucleus
- have thicker, bilamellar walls
-
- 3. Mature sporangia
- - hallmark of R. seeberi infection
- average 100-300 microns diameter
- "zonation" of mature and immature endospores
- mature endospores have "eosinophilic globular bodies"
Little is known about the epidemiology of this organism. Although
generally considered to be a fungus, its taxonomy is still uncertain
and it has yet to be cultivated on synthetic media. R. seeberi
typically induces the formation of chronic inflammatory nasal
polyps. Conjunctival lesions have been reported, but these are
far less common than respiratory lesions.
- This organism has been studied most intensively in man, although
animal cases have been documented and described in horses, mules,
cattle, dogs, a goose, 2 ducks and, most recently, in 41 swans
from a lake in central Florida. This outbreak lends support to
the hypothesis that R. seeberi may be an aquatic organism.
-
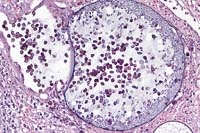
Case 30-1. Skin. A mature sporangium of Rhinospordium
seeberi is discharging some of its endospores to surface of the
skin. A small portion of an immature sporangium is at the edge.
It contains only floccular eosinophilic material. 20X
AFIP Diagnosis: Stratified squamous epithelium and loose
connective tissue: Inflammatory polyp, with moderate chronic-active
inflammation, multifocal hemorrhage, epithelial necrosis, and
multiple fungal sporangia, pink river dolphin (Inia geoffrensis),
cetacean, etiology consistent with Rhinosporidium seeberi.
Conference Note: Based only on the morphology of the
infecting organism, the differential diagnosis might include coccidioidomycosis
and adiasporomycosis. However, the spherules of Coccidioides immitis
are generally smaller than rhinosporidial sporangia, and they
contain endospores that are generally the same size and shape
throughout the spherule. Chrysosporium parvum, the etiologic agent
of adiasporomycosis, is larger than Rhinosporodium, has a thicker
wall, and does not reproduce by endosporulation.
Rhinosporidiosis occurs sporadically worldwide, but is hyperendemic
in India, Sri Lanka, and Southeast Asia.1,2 Infection is associated
epidemiologically with rural and aquatic environments. A recent
study from India suggests that Rhinosporidium seeberi is a form
of the cyanobacterium Microcystis aeruginosa, which was isolated
from water samples in which human patients with rhinosporidiosis
were bathing.8
Contributor: Wildlife Conservation Society, Department
of Pathology, 185th St. and Southern Blvd., Bronx, NY 10460
- References:
- 1. Chandler FW, Kaplan W, Ajello L: Color Atlas and Text
of the Histopathology of Mycotic Diseases. Year Book Medical
Publishers, Inc. Chicago. pp. 109-111, 1980.
- 2. Chandler FW, Watts JC: Pathologic Diagnosis of Fungal
Infections. ASCP Press, Chicago. pp. 27-33, 1987.
- 3. Cheville NF: Ultrastructural Pathology. An Introduction
to Interpretation. Iowa State University Press. Ames, IA. pp.
775-776, 1994.
- 4. Gaines JJ, Clay JR, Chandler FW, Powell ME, Sheffield
PA, Keller III AP: Rhinosporidiosis: three domestic cases. Southern
Medical Journal 89(1):65-67, 1996.
- 5. Kennedy FA, Buggage RR, Ajello L: Rhinosporidiosis: A
description of an unprecedented outbreak in captive swans (Cygnus
spp.) and a proposal for revision of the ontogenic nomenclature
of Rhinosporidium seeberi. Journal of Medical and Veterinary
Mycology 33:157-165, 1995.
- 6. Kwon-Chung KJ: Phylogenetic spectrum of fungi that are
pathogenic to humans. Clinical Infectious Diseases 19 (Suppl
1): S1-7, 1994.
- 7. Levy MG, Meuten DJ, Breitschwerdt EB: Cultivation of Rhinosporidium
seeberi in vitro: interaction with epithelial cells. Science
234:474-476, 1986.
- 8. Ahluwalia KB, Maheshwari N, Deka RC: Rhinosporidiosis:
a study that resolves etiologic controversies. Am J Rhino 11(6):479-483,
1997.
International Veterinary Pathology Slide Bank:
Laser disc frame #6637, 6638, 6639, 8246, 4471, 14472, 14473
Case II - 94-114 (AFIP 2453724)
Signalment: 8-week-old, female, New Zealand White, rabbit.
History: Incidental finding in a rabbit infected with
Campylobacter jejuni.
Gross Pathology: None.
Contributor's Diagnosis and Comments: Kidney: Nephritis,
interstitial, chronic, moderate, with gram positive intraepithelial
protozoal organisms - Etiology consistent with Encephalitozoon
cuniculi.
Encephalitozoon cuniculi is an occasional, usually asymptomatic,
parasite of rabbits. The primary importance of the organism in
rabbits is interference with the interpretation of experimental
data. In other species (dogs, cats, and wild carnivores) the organism
causes clinical, often fatal encephalitis and nephritis.
The organisms are most commonly found in the renal tubular
epithelial cells and capillary endothelial cells within the central
nervous system.
- Differentiating Encephalitozoon cuniculi from toxoplasmosis
can be accomplished a number of ways. Encephalitozoon stains
poorly on H&E, is gram positive, and its spores are birefringent;
Toxoplasma stains well on H&E, stains poorly with Gram stains,
and is not birefringent.
-
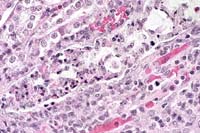
- Case 30-2a. Kidney. Tubule whose lumen is filled with
necrotic epithelial cells that contain numerous but vague eosinophilic
organisms in the cytoplasm. 40X
-
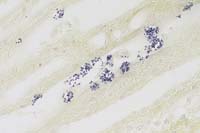
- Case 30-2b. Kidney. Numerous microsporidian organisms
(Encephalitozoon cuniculi) in a degenerating tubule. Gram. 40X
AFIP Diagnosis: Kidney: Nephritis, tubulointerstitial,
acute to chronic, multifocal, moderate, with tubular dilatation,
tubular epithelial necrosis, and intracellular and extracellular
protozoa, New Zealand white rabbit, lagomorph.
Conference Note: Transmission of Encephalitozoon cuniculi,
a microsporidian, is primarily via ingestion of urine containing
the infective spores. Transplacental infection has also been reported.3
In addition to the susceptible species listed above, infection
occurs in rats, mice, guinea pigs, hamsters, and humans.3 At least
three strains of E. cuniculi have been identified based on host
specificity and other criteria.4
Encephalitozoon cuniculi infection of mice is used as a model
of human microsporidiosis. Mouse strains differ greatly in their
susceptibility to infection, with C57BL/6, DBA/1, and 129J being
highly susceptible, and BABL/c, A/J, and SJL strains being relatively
resistant.4 Athymic (nu/nu) mice experience high mortality with
infection.
Contributor: Naval Medical Research Institute, Pathobiology
Division, 8901 Wisconsin Avenue, Bethesda, MD 20889-5607
- References:
- 1. Szabo JR, Shadduck JA: Experimental encephalitozoonosis
in neonatal dogs. Vet Pathol 24:99-108, 1987.
- 2. Cutlip RC, Beall CW: Encephalitozoonosis in arctic lemmings.
Lab Anim Sci 39(4):331-333, 1989.
- 3. Soulsby EJL: Helminths, Arthropods and Protozoa of Domesticated
Animals, 7th edition, Lea & Febiger, Philadelphia, pp. 742-743,
1982.
- 4. Baker DG: Natural pathogens of laboratory mice, rats,
and rabbits and their effects on research. Clinical Microbiology
Reviews 11(2):231-266, 1998.
International Veterinary Pathology Slide Bank:
Laser disc frame #5278-5281, 19463.
Case III - Mississippi State University (AFIP 2376327)
Signalment: Adult, female, cottontail rabbit (Sylvilagus
floridanus)
History: This animal was found alive but weak, depressed,
thin, and easily captured.
Gross Pathology: The animal was emaciated, flea infested,
and had several ticks. The peritoneal cavity contained approximately
10 ml of serous fluid. The spleen was 2x2x6 cm, and multiple disseminated
pinpoint to 1 mm white foci were visible on the capsular and cut
surfaces. The liver had similar foci. Mesenteric, hilar and mediastinal
lymph nodes were enlarged, gray, and friable. Several tapeworm
cysts were noted in the peritoneal cavity and attached to the
liver and pleura.
Laboratory Results: Francisella tularensis was isolated.
Contributor's Diagnosis and Comments: Disseminated multifocal
and coalescing necrotizing splenitis.
- This case represents a classic case of tularemia. Care should
be taken by prosectors as this condition is zoonotic.
-
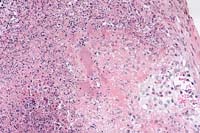
- Case 30-3. Spleen. There is extensive liquefactive
necrosis of paranchyma with vague, basophilic, intralesional
bacterial colonies (center). 20X
- AFIP Diagnosis: Spleen: Splenitis, necrotizing, acute
to subacute, multifocal to coalescing, severe, with necrotizing
vasculitis, fibrin thrombi, and numerous colonies of coccobacilli,
cottontail rabbit (Sylvilagus floridanus), lagomorph.
Conference Note: Gram stains demonstrated that the coccobacilli
are gram-negative.
Tularemia (deer fly fever, rabbit fever) is a zoonotic disease
with worldwide distribution, affecting more than 100 species of
wild and domestic mammals, birds, fish, and reptiles. It is primarily
a disease of wild rabbits and rodents and -shares many key features
with endemic plague, with which it was originally confused in
a 1911 outbreak in ground squirrels in Tulare County, California.
There are two antigenically similar strains of F. tularensis:
1. F. tularensis subsp. tularensis (Type A) occurs only in
North America, where it is the most frequently isolated strain
(70% of human cases). It is associated with tick borne tularemia
in rabbits, and produces the classic disease in humans.
2. F. tularensis subsp. palaearctica (Type B) occurs throughout
the world (except Australia and Antarctica), and is less virulent
than Type A. It is associated with mosquitoes and rodents, and
is frequently linked to waterborne disease of rodents, particularly
beavers and muskrats.
Transmission of the disease may occur by a variety of routes
including direct contact of the organism with intact or abraded
skin or mucous membranes, ingestion or inhalation of the organism,
or by percutaneous inoculation via arthropod vectors. As few as
10 bacilli may induce disease when inhaled or injected, whereas
a much larger dose is required for oral infection.
Ticks are reported most frequently as the source of human infection
in the United States, followed by rabbits. Two seasonal peaks
are associated with tularemia: one during tick season, the other
during the hunting season, associated with contact with infected
rabbits. Dermacentor variabilis (dog tick), D. andersoni (wood
tick), and Amblyomma americanum (lone star tick) are considered
the most important tick vectors in North America. Both transstadial
and transovarian passage of F. tularensis have been documented,
making the tick both a vector and a reservoir of infection. The
deerfly, Chrysops discalis, is also an important vector in North
America.
Clinical signs are those of an acute septicemia and vary with
the route of infection, the strain of the organism, and the species
involved. Rabbits and rodents are often found dead without premonitory
signs. In cats, tularemia is associated with nonspecific clinical
signs, including pyrexia, anorexia, lethargy, lymphadenopathy,
oral ulcers, hepatomegaly, and icterus.2
Contributor: Mississippi State University, College of
Veterinary Medicine, Mississippi State, MS 39762
- References:
- 1. Davidson WR, Nettles VF: Field Manual of Wildlife Diseases
in the Southeastern United States. Southeastern Cooperative Wildlife
Disease Study, pp 212-215, 1988.
- 2. Woods JP, Crystal MA, Morton RJ, Panciera RJ: Tularemia
in two cats. JAVMA 212(1):81-83, 1998.
- 3. Valli VEO: The hematopoietic system. In: Pathology of
Domestic Animals, 4th edition, Jubb KVF, Kennedy PC, Palmer N
(eds.), Academic Press, Inc, vol. 3, pp. 244-245, 1993.
International Veterinary Pathology Slide Bank:
Laser disc frame #3476, 3477, 5307, 5308, 11121-11124, 22253.
Case IV - 90-4468 (AFIP 2327342)
Signalment: 2-year-old, male, Doberman Pinscher, canine,
named "Mr. Blue".
History: This dog had a history of chronic keratoconjunctivitis
and generalized bilaterally symmetrical hair loss.
Gross Pathology: Bilaterally symmetrical hair loss.
Laboratory Results: Antinuclear antibody: 1:20
Baseline T4: 0.2 (normal 1.0-4.0)
Contributor's Diagnoses and Comments:
Haired skin: Diffuse superficial and follicular orthokeratotic
hyperkeratosis
Multifocal follicular atrophy with melanin clumping
Multifocal, perifollicular melanophage accumulation
Mild superficial perivascular hyperplastic dermatitis
Condition: Color mutant alopecia.
- These sections demonstrate the classic histologic features
of color mutant alopecia in the dog: diffuse superficial and
follicular orthokeratotic hyperkeratosis; dilated keratin-filled
follicles; multiple melanin aggregates within hair shafts and
bulbs; occasional fractured hairs; diffuse, moderate adnexal
atrophy; and scattered, generally perifollicular, deep dermal
and pannicular aggregates of melanophages. In this case, there
is a minimal superficial perivascular dermatitis with mild epidermal
hyperplasia. Most sections have one or more anagen hair follicles,
though many follicles are atrophied. Some sections contain intrafollicular
accumulations of inflammatory cells. Arrector pili muscles are
present, but not enlarged or vacuolated. The low baseline T4
level might indicate, but is not diagnostic for, hypothyroidism.
There are no histologic changes to suggest hypothyroidism and
it is unknown if the dog was on thyroid medication at the time
of biopsy. Besides the blue Doberman, this condition can be seen
in fawn Irish Setters, red and fawn Dobermans, and other "blue"
varieties of various breeds.
-
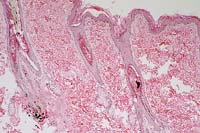
- Case 30-4. Skin. There is hair follicle atrophy with
clumping of melanin in one follicle accompanied by mild intrafollicular
hyperkeratosis. 4X
- AFIP Diagnosis: Haired skin: Follicular atrophy, ectasia,
and hyperkeratosis, diffuse, moderate, with intrafollicular melanin
clumping, peribulbar melanophages, and mild multifocal superficial
lymphoplasmacytic and eosinophilic dermatitis, Doberman Pinscher,
canine.
Conference Note: This hereditary syndrome is associated
with a color-dilution gene, but it is not known if the gene is
directly responsible for initiating the skin disease or if a linked
gene codes for the associated follicular changes.2
Clinically, this disease is characterized by a gradual onset
of a dry, dull, brittle, poor-quality hair coat. Hair shafts break,
and regrowth is often poor. Follicular papules and comedones may
develop, and chronic cases may exhibit hyperpigmentation.
The clinical differential diagnosis of color mutant alopecia
should include other generalized atrophic or dysplastic diseases
affecting the hair follicle including hypothyroidism, hyperadrenocorticism,
canine follicular dysplasia, and acquired pattern alopecia.2
Contributor: Department of Pathology, Cornell University,
Ithaca, NY 14853
- References:
- 1. Brignac MM, Foil CS, Al-Bagdadi FAK, Kreeger J: Microscopy
of color mutant alopecia. Ann Meet Am Acad Vet Dermatol and Am
Coll Vet Dermatol., pp. 14-15, 1988.
- 2. Gross TL, Ihrke PJ, Walder EJ: Veterinary Dermatopathology.
A macroscopic and microscopic evaluation of canine and feline
skin disease. Mosby Year Book, St. Louis, MO, pp. 298-301, 1992.
- 3. Scott DW, Miller Jr WH, Griffin CE: Muller & Kirk's
Small Animal Dermatology, 5th edition, W.B. Saunders Company,
Philadelphia, pp. 777-779, 1995.
International Veterinary Pathology Slide Bank:
Laser disc frame #11787, 11856, 11857, 13848-13851.
Terrell W. Blanchard
Major, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: blanchard@email.afip.osd.mil
* The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
Return to WSC Case Menu.

