Signalment: 3-year-old, male, rhesus macaque (Macaca mulatta).
History: This animal was inoculated with a pathogenic molecular clone of the simian immunodeficiency virus (SIVmac239) eight months previously. The animal developed progressive anorexia and weight loss. Mild ascites and edema of the legs and scrotum were detected clinically and the animal was euthanized.
Gross Pathology: The gall bladder and common bile duct were thickened, white and edematous. The gallbladder contained 3cc of serous fluid. The liver was yellow-tan with a marked reticular pattern and rounded edges.
Laboratory Results: Serum chemistry at the time of death revealed elevations in total bilirubin (6.23mg/dl; normal 0.00-0.39mg/dl), alkaline phosphatase (22,630U/L; normal 0-704U/L), ALT (79U/L; normal 0-59U/L), AST (97U/L; normal 0-46U/L), and hypoalbuminemia (1.3g/dl; normal 3.3-4.7g/dl).
Contributor's Diagnosis and Comments: Liver: Nonsuppurative cholangiohepatitis with marked proliferation of bile ductules, bridging portal fibrosis and extrusion of individual biliary epithelial cells containing microsporidial organisms.
These findings are diagnostic of infection with Enterocytozoon bieneusi. First recognized in biopsy specimens from human AIDS patients in 1985, E. bieneusi is the most commonly diagnosed microsporidium in man. This obligate intracellular protozoan has been identified in 30% to 50% of HIV-infected patients with chronic unexplained diarrhea, and likely represents a significant cause of wasting and malabsorption. More recently, E. bieneusi has been found in association with hepatobiliary and pulmonary lesions in human AIDS patients and with diarrhea in HIV-negative immunocompetent individuals. Hepatobiliary infection of AIDS patients may result from contiguous spread from the gastrointestinal tract and is linked with papillary stenosis, acalculous cholecystitis, bile duct dilatation, and sclerosing cholangitis. Despite its relatively common occurrence in human AIDS patients, basic aspects of parasite biology, epidemiology, and host immunity remain poorly understood.
Infection with E. bieneusi has been diagnosed in three species of macaques (Macaca mulatta, M. cyclopis and M. nemestrina) and the organism may be identified in both immunocompetent and immunodeficient individuals. This organism is virtually indistinguishable at the morphologic and genetic level from that identified in human AIDS patients. In immunodeficient animals, the organism is most commonly found in the gall bladder and common bile duct and less frequently in the small intestine and liver. The characteristic findings in the gall bladder and common bile duct are a nonsuppurative and proliferative cholecystitis and choledochitis. As seen in this case, extrusion of individual biliary epithelial cells is often evident. These cells contain mature spores which are visible as 1.0-1.5 um diameter negative images or lightly basophilic bodies on routine H&E stained sections. In our colony, the triad of bridging portal fibrosis, nodular lymphocytic infiltrates and marked bile duct and ductular hyperplasia within the hepatic parenchyma has been found exclusively in association with E. bieneusi infection. In these cases, the organism is found most commonly in intralobular hepatic ducts and less frequently within proliferating bile ductules.
In a survey based on PCR amplification of enterocytozoon DNA from feces, E. bieneusi has been diagnosed in 17% of immunocompetent rhesus macaques housed under conventional conditions. Animals are in general asymptomatic but may be persistently infected for periods exceeding 12 months. In these persistently infected animals, the organism becomes localized to the hepatobiliary tree where it may be associated with a nonsuppurative choledochitis.
Due to the small size of the spores, recognition of E. bieneusi
in sections may be difficult. Weber's modified trichrome, Ziehl-Neelsen
acid fast, Wright Giemsa and Brown-Hopps gram stain may all be
utilized to help visualize the organism. In situ hybridization
utilizing a probe directed at the small subunit ribosomal RNA
has proven to be a sensitive and specific indicator of E. bieneusi
infection and in general reveals far more organisms than conventional
histochemical techniques. The increased sensitivity of in situ
hybridization is not unexpected. We suspect that while conventional
techniques rely on specific anatomic structures, such as the thick
exospore wall, to demonstrate organisms, in situ hybridization
detects RNA that should be present in all parasite life stages.
By in situ hybridization, plasmodial stages stain as 8-15mm diameter
round to oval bodies located within the host cell cytoplasm. These
plasmodia are often in a perinuclear location and contain small
nonstaining clefts.
Ultrastructural examination may also be used to diagnose infection
and to distinguish E. bieneusi from other microsporidia commonly
seen in AIDS patients. Findings characteristic of E. bieneusi
include: 1) electron lucent inclusions which are present throughout
the life cycle, 2) direct contact with the host cell cytoplasm,
3) elongated nuclei during early development, 4) precocious development
of the polar tube complex, 5) late thickening of the sporogonial
plasmodium membrane, 6) formation of electron dense discs during
sporogony, and 7) presence of polar tube doublets visible as 5-7
coils in section. These features are unique to E. bieneusi and
the organisms identified in human and macaque tissue are morphologically
indistinguishable. Moreover, DNA sequence data from E. bieneusi
derived from both rhesus macaques and human AIDS patients reveals
extensive identity over the large and small subunit ribosomal
gene complex.
The diagnosis in the case presented was confirmed by in situ hybridization, polymerase chain reaction and ultrastructural examination. Concurrent infection with Cryptosporidium parvum or rhesus cytomegalovirus, both common causes of cholangitis in SIV-inoculated macaques, was ruled out by immunohistochemistry and in situ hybridization, respectively. Bacteriologic culture of the bile obtained at necropsy was negative.
Conference Note: In specially-stained sections viewed in conference, the Brown and Brenn gram stain was found to mildly enhance visibility of the organisms in extruded biliary epithelial cells. Although other pathogenic microsporidia are commonly acid-fast, these organisms did not stain acid-fast by the Ziehl-Neelsen method used at the AFIP.
Signalment: 4-month-old, female, Holstein-Friesian, bovine.
History: This calf presented with a 3 month history of scours, coughing, respiratory difficulty, skin problems, eye discharge, and weakness. On physical exam, the animal appeared depressed, with a general loss of body condition. She was observed to urinate 4 times in a 25 minute period and pass very loose feces. Temperature, pulse, and respiration were normal. Superficial lymph nodes were enlarged.
Gross Pathology: There was staining of the perineal
region with diarrheic feces. There were multiple foci of scaling,
crusting, erythema, and alopecia of the nonpigmented areas of
skin of the dorsal aspect of the body, especially at the thoracolumbar
region. The thymus was 1/5 to 1/10 normal size. All of the mesenteric
lymph nodes were enlarged 2 to 3 times normal, and there was mild
thickening of the small intestinal mucosa.
Laboratory Results:
Virology: Negative for bovine virus diarrhea virus, infectious
bovine rhinotracheitis virus, bovine adenovirus 3, bovine adenovirus
5, rotavirus, and coronavirus.
Bacteriology: Negative for Salmonella spp. and Campylobacter spp.
Parasitology: Large numbers of Eimeria spp. oocysts.
Contributor's Diagnosis and Comments: Skin, dermatitis, superficial, suppurative, multifocal, severe with intralesional Dermatophilus organisms.
Conference Note: Dermatophilus congolensis belongs to the order Actinomycetales, and is a gram-positive, pleomorphic, facultative anaerobic bacterium characterized by narrow, branching filaments with transverse and longitudinal septation giving rise to parallel rows of coccoid cells (zoospores). Dermatophilosis is diagnosed in cattle, sheep, goats, horses, wild ungulates, and rarely in dogs, cats, non-human primates, reptiles, birds, pigs, and man. Zoospores are able to survive in dried exudate on animals for months, but do not survive well on soil or inert objects. Dermatophilus congolensis has world-wide distribution. It is endemic in the tropical and subtropical parts of Africa, South America, Australia, New Guinea, New Zealand, and India, and is sporadic in the United States, the United Kingdom, Europe, and Canada. Economic losses are substantial in endemic areas, due to hide damage, deterioration of general condition, and loss of meat and milk production. Dermatophilosis may predispose to myiasis and secondary bacterial infections, and the organism can coinfect lesions of poxviral disease.
Principal sources of infection are affected animals and healthy carriers, which often have occult colonies in the ostia of hair follicles. Infection can only occur if epidermal integrity is compromised either through trauma or from prolonged wetting. Moisture causes keratinocytes to swell and detach from each other, and intense wetting leaches out the protective lipid film from the intercellular spaces of the stratum corneum. Arthropod vectors, thorny pastures, and shearing wounds predispose to infection. Favorable moisture and temperature activate latent zoospores, and those recently acquired from other animals or arthropod vectors. Motile zoospores migrate chemotactically to areas of the susceptible host's skin from which the respiratory efflux of CO2 is highest (thin skinned areas). Zoospores germinate to produce hyphae which penetrate the living epidermis but do not usually penetrate the basement membrane zone.
Bacterial invasion stimulates an acute inflammatory response, characterized by superficial dermal edema, and accumulation of large numbers of neutrophils. Neutrophilic exocytosis leads to microabscess formation in the epidermis and the external root sheath. The invaded epidermis prematurely cornifies; within 24 hours postinfection, epidermal regeneration from the adjacent root sheath is established, and by 36 hours a new epidermis has formed under the exudate. Dermatophilus congolensis may cyclicly invade the newly formed epidermis, and stimulate additional bursts of dermal inflammation resulting in an increasingly thick scab composed of alternating layers of ortho- and parakeratosis and degenerating neutrophilic exudate. Any area of skin can be infected, and there is no breed, age or sex predilection.
Contributor: Department of Veterinary Pathology, Louisiana State University, School of Veterinary Medicine, Baton Rouge, LA 70803
International Veterinary Pathology Slide Bank:
Laser disc frame #3565, 4053, 4054, 4432-4435, 12093, 12862, 12863,
13119, 22592-22595, 23262.
Signalment: Tissue from a one-year-old castrated male Dorset cross lamb experimentally inoculated intradermally with sheep pox virus.
History: One week postinoculation, the animal became febrile and developed dermal nodules multifocally. There was severe depression and considerable dyspnea. The animal died.
Gross Pathology: Significant lesions were found in the skin and lungs. There was an extensive distribution of dermal nodules, some of which had necrotic centers and were very excoriating in appearance. The lungs were disrupted by multifocal areas of edema and consolidation, very prominently seen in subpleural zones.
Contributor's Diagnosis and Comments: Dermatitis, subacute to chronic, severe, with vasculitis and "sheep pox cells" (etiology: capripoxvirus, specifically sheep pox virus).
Sheep pox is an acute, systemic, contagious disease of sheep caused by a member of the Capripoxvirus genus of the family Poxviridae. Sheep pox virus shares this genus with the viruses of goat pox and lumpy skin disease. Sheep pox is a serious problem throughout Africa and much of Asia.
The most striking clinicopathologic feature is generalized cutaneous eruptions. Each of these occupies the full thickness of the skin. Often centers become necrotic and ulcerated, resulting in a "sitfast". A high percentage of affected animals may develop pneumonia, which grossly appears as multiple foci of subpleural consolidation and edema. In addition, pox lesions can sometimes be found on many serosal surfaces.
Conference Note: Transmission of sheep pox virus (SPV) is by direct contact, aerosol, or fomites. Insect transmission has been demonstrated experimentally3, but viral replication does not occur in vectors. SPV is resistant to drying and can remain viable for several months in wool or dried crusts. The incubation period is 8-12 days or longer. Morbidity ranges from 5-80% in adult sheep; in lambs and kids under one month of age, morbidity may approach 100%4. Mortality ranges from 5-50% in adults, but in lambs and kids it may be as high as 95%4. Merino sheep are particularly susceptible, whereas breeds native to endemic areas are more resistant.3
Following introduction of SPV into a susceptible host, there is local viral replication in the epidermis, dermis, or respiratory epithelium. Infected macrophages migrate to local lymph nodes, where further viral replication occurs. There is extensive lymphoid proliferation in lymph nodes. A primary cell-associated viremia causes viral spread to other organs, i.e. spleen, liver, and lung. A secondary viremia follows and leads to disseminated skin lesions. During lesion development, SPV causes extensive vasculitis, thrombosis, and necrosis.
Contributor: Foreign Animal Disease Diagnostic Laboratory, U.S. Dept. of Agriculture, Animal and Plant Health Inspection Service, P.O. Box 848, Greenport, NY 11944
Signalment: 5-month-old, male, lemur (Lemur macaco).
History: Two young Lemur macaco were imported from Madagascar to the Parc Zoologique de Paris at 4 months of age. The first one died two weeks after its arrival due to severe diarrhea. Two weeks later, the second lemur also developed a profound diarrhea and was subsequently euthanatized. Samples from the gastrointestinal tract were sent for bacteriology, parasitology, mycology and histopathology. Laboratory results were negative.
Gross Pathology: Over ten centimeters, the proximal part of the colon was markedly thickened and firm like a pipe tube.
Contributor's Diagnosis and Comments: Colon: Severe, chronic, locally extensive to diffuse, ulcerative, necrotizing transmural colitis with intraluminal and intralesional amoebic organisms and intraluminal trichomonad organisms.
Histologically, the lesions consist of severe, multifocal to coalescing ulceration of the colonic mucosa and abscess formation within muscular layers of the colon. Intramural abscesses are composed of large mineralized necrotic centers and rims of active trophozoites of amoebic protozoa (Entamoeba histolytica). These trophozoites are located at the limit between necrotic and viable tissue. Abscesses are surrounded by granulation tissue infiltrated by neutrophils and lymphocytes. Trophozoites are fairly large (between 30 to 60 mm) and often have food vacuoles which contain red blood cells and a central spherical nucleus. A communication between abscesses and ulcerations of the mucosa is readily visible in many sections. The wall of the opening is lined by a layer of trophozoites and necrotic debris. Mineralized cellular debris intermingled with trophozoites and bacterial colonies cover or replace most of the colonic mucosa. In a number of sections, small, elongate, about 8 mm long, basophilic unicellular organisms are observed in the necrotic debris covering the mucosa and are interpreted as Trichomonas sp.
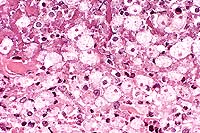
Conference Note: In contrast to Old World primates, which often harbor E. histolytica without host tissue invasion, New World species do not routinely harbor the organism. Tissue invasion and disease from E. histolytica are more likely in New World monkeys, especially those suffering poor nutritional status, stress or immunologic compromise.
The life cycle of E. histolytica is direct, with fecal-oral transmission via feces-contaminated water, food, cleaning and feeding utensils, or the immediate environment. Vectors include roaches, houseflies, ticks, mites, and mice. Infection begins when infective stage amoeba cysts are ingested. In the ileum, excystment takes place. Nuclear and cytoplasmic division yields up to eight small uninucleate metacystic amoebae. Amoebae grow and feed on bacteria, tissue debris, and erythrocytes. The trophozoites (typical amoeboid or motile forms) move down the intestinal tract, dividing by binary fission; these may invade colonic epithelial cells. Precysts form when the trophozoite slows its metabolic activity, extrudes or digests cytoplasmic food particles, shrinks and rounds up. It secretes a chitinous cell wall that will thicken with cyst maturation. Cysts form in the gut lumen. All three stages (trophozoite, precyst, and cyst) of the organism may be found in the feces. The predominant form is dependent on the disease stage; trophozoites are shed during acute dysentery or diarrhea, whereas cysts are usually associated with formed stools and will result in transmission. Trophozoites released in the feces rapidly desiccate outside the host; however, the infective stage cysts are resistant and survive in the environment and upper gastrointestinal tract.
Another significant pathogenic species of Entamoeba is E. invadens, which causes fatal disease in numerous reptilian species. In addition to gastrointestinal lesions, E. invadens can enter lymphatics and venules and can travel to, and cause amoebic abscesses in, the liver, lungs, central nervous system, spleen, pancreas, kidneys, and subcutis.
Contributor: Pfizer Centre de Recherche, ZI Pocé-sur-Cisse, BP 159, Amboise Cedex, France 37 401
International Veterinary Pathology Slide Bank:
Laser disc frame #7249, 7250, 11263, 12339, 23334
Terrell W. Blanchard
Major, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: blanchard@email.afip.osd.mil
* The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry