Results
AFIP Wednesday Slide Conference - No. 1
1997
Conference Moderator: COL William Inskeep, II
Diplomate, ACVP
Chairman, Department of Veterinary Pathology
Armed Forces Institute of Pathology
Washington, D.C. 20306-6000
Return to WSC Case Menu.
Case I - I97-4346 (AFIP 2594302)
-
- Signalment: Wild muscovy ducks.
History: Several ducks were found dead around a lake.
Gross Pathology: Necropsy conducted on several ducks revealed
moderate autolysis and no gross lesions.
Laboratory Results: N/A
- Contributor's Diagnoses and Comments:
- 1. Multifocal hepatic necrosis with intranuclear inclusions.
- 2. Fibrinohemorrhagic and necrotizing enteritis.
3. Fibrinonecrotic and ulcerative esophagitis with intranuclear inclusions.
- The lesions are consistent with duck viral enteritis, a herpes virus
infection. This submission includes tissues from two different birds. The
liver on most slides has discrete areas of necrosis and numerous intranuclear
eosinophilic inclusions in hepatocytes that are not necrotic. The liver
on some slides has diffuse congestion with areas of hemorrhage, necrosis
and fibrin with abundant bacteria and no inclusions. The esophagus in most
slides has complete ulceration with occasional inclusions in the glandular
epithelium. On a few slides some mucosa is left which contains eosinophilic
cytoplasmic inclusions that may be pox virus. A few slides also have Capillaria
in the necrotic mucosa.
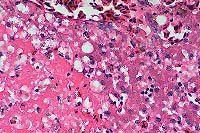
- Case 1: Liver. Note focus of coagulative necrosis, mild infiltration
of lymphocytes and plasma cells, and scattered hepatocytes containing eosinophilic
intranuclear inclusions.(40x obj)
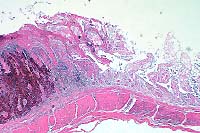
- Case 1: Esophagus. Necrotic mucosa and submucosal glands layered with
basophilic bacteria, multifocal clusters of bipolar plugged nematode eggs.
(20x obj)
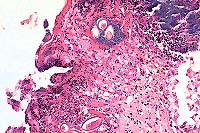
- Case 1: Small Intestine. Demonstrates focal hemorrhage expanding lamina
propria with sloughing & necrosis of mucosal epithelium.(4x obj).
- AFIP Diagnoses:
- 1. Liver: Hepatitis and cholangitis, necrotizing, multifocal, random,
acute, mild, with eosinophilic intranuclear inclusion bodies, Muscovy duck,
avian, etiology consistent with duck herpesvirus 1.
- 2. Esophagus: Esophagitis, necrotizing, acute, diffuse, severe, with
myriad bacilli and cocci and eosinophilic intranuclear and intracytoplasmic
inclusion bodies, etiology consistent with duck herpesvirus 1.
3. Esophagus, mucosa: Intra-epithelial nematode eggs, numerous, etiology
consistent with Capillaria sp.
4. Small intestine: Enteritis, necrotizing, acute, diffuse, severe, with
hemorrhagic necrosis of lymphoid tissue.
Conference Note: Duck virus enteritis (DVE) is a highly contagious
and often fatal alpha-herpesviral infection of ducks, geese, and swans.
This disease is enzootic in North America and has been reported in Europe,
Asia, and India. DVE can be transmitted by direct contact, or indirectly
by contact with a contaminated environment. Experimentally, it can be transmitted
via oral, intranasal, intravenous, intraperitoneal, intramuscular, and cloacal
routes. Potential transmission by bloodsucking arthropods may be possible
during viremia. A carrier state has been suspected in wild ducks.
In domestic ducks, the incubation period ranges from 3-7 days. Once overt
signs appear, death usually follows within 1-5 days. Clinical signs in domestic
breeders include sudden, high, persistent flock mortality; 25-40% drop in
egg production; photophobia, inappetence, extreme thirst, droopiness, ataxia,
ruffled feathers, nasal discharge, soiled vents, and watery diarrhea. In
young ducklings, dehydration, weight loss, cyanosis of the beak, and bloodstained
vents are often observed. Total mortality has ranged from 5-100%.
Gross lesions of DVE include: hemorrhage in or on the myocardium, endocardium,
intestinal lumen (including the classic ‘annular band hemorrhage'),
and surfaces of liver, pancreas, intestine, lungs, and kidney; oral, esophageal,
and intestinal erosions with mucosal sloughing; reddening of the bursa of
Fabricius; and irregularly distributed petechiae and white foci over the
surface of the liver.
Histologic lesions include gastrointestinal necrosis, hepatocellular
necrosis, necrosis and loss of lymphoid tissue, and necrotizing vasculitis.
As in this case, eosinophilic intranuclear viral inclusion bodies are present
in hepatocytes, biliary epithelium, gastrointestinal epithelium, and lymphoreticular
cells associated with these necrotizing lesions. Intranuclear inclusions
have also been reported in vascular endothelium. As the contributor observed,
some of the esophageal epithelial cells contain eosinophilic intracytoplasmic
inclusion bodies. While these inclusions may be poxviral inclusions, conference
participants noted that intracytoplasmic herpesviral inclusions have been
reported in a natural outbreak of DVE in muscovy ducks.2
Two species of Capillaria, C. contorta and C. annulata, are found in
the crop and esophagus of birds, though some consider these species to be
synonymous. The life cycle is either direct or indirect, in which case the
intermediate host is an earthworm. Light infections produce only mild inflammation
and thickening of the crop. In heavy infections there is a marked thickening
of the crop and esophageal wall, a marked catarrhal inflammation, and sloughing
of the mucosa. The birds are emaciated and weak.
Contributor: Department of Biomedical Sciences and Pathobiology,
College of Veterinary Medicine, Virginia Tech, Blacksburg, VA 24061-0442.
References:
- 1. Leibovitz L: Duck Viral Enteritis. In: Diseases of Poultry, 9th
ed., BW Calnek, ed. Iowa State University Press, Ames, IA;pp. 609-618,
1991.
- 2. Barr BC, Jessup DA, Docherty DE, Lowenstine LJ: Epithelial intracytoplasmic
herpes viral inclusions associated with an outbreak of duck virus enteritis.
Avian Diseases 36:164-168, 1992.
- 3. Gerlach H: Viruses. In: Avian Medicine: Principles and Application,
Ritchie, Harrison, and Harrison, eds. Wingers Publishing, Inc; pp. 877-8,
1994.
- 4. Soulsby EJL. Helminths, Arthropods, and Protozoa of Domisticated
Animals, 7th ed., Lea and Febiger; pp. 338-339, 1982.
International Veterinary Pathology Slide Bank:
Laser disc frame #19320, 19321, 20511, 20512
Case II - 691-97 (AFIP 2595292)
Signalment: 1-month-old female Beefmaster calf.
History: This calf was one of two reported by the owner to be
lethargic, with swollen joints and loss of hair coat. The animal was suspected
to have navel ill. A second calf was presented 4 weeks later with similar
history and gross findings described below.
Gross Pathology: There was bilateral renal hypoplasia, characterized
by an irregular, thin and fibrotic cortex, and dilated renal calyces filled
with granular and gritty to mucoid material.
Laboratory Results: None available
Contributor's Diagnosis and Comments: Nephron loss, degeneration
and necrosis, stromal fibrosis, diffuse, severe, oxalate crystals and chronic
tubulointerstitial nephritis.
Gross and histopathologic findings, age, and breed of the calves are
diagnostic for congenital/primary renal oxalosis, a condition similar to
those reported in humans as an inherited metabolic disease. Secondary renal
oxalosis in any age ruminant can be precipitated due to ingestion of plants
containing high levels of oxalate, a circumstance ruled out in these two
calves.

Case 2. Kidney. Note refractile crystalline material replacing tubular
epithelium.

Case 2 Kidney. Polarized light. Note anisotropic crystals within degenerate
tubules and interstitium.
AFIP Diagnosis:
Kidney: Degeneration, necrosis, and loss, tubular, diffuse, severe, with
intraluminal oxalate crystals, diffuse moderate interstitial fibrosis, and
multifocal mild lymphocytic interstitial nephritis, Beefmaster, bovine.
Conference Note: Primary oxaluria is a general term for inherited
metabolic disorders resulting in severe renal oxalosis and early death caused
by renal failure. In human beings, three types are described. Types I and
II are disorders of glyoxylate metabolism, and are inherited as autosomal
recessive traits. A third type appears to be a primary defect in oxalate
absorption.
As the contributor noted, plants are the usual source of oxalate poisoning
in ruminants. Important oxalate-accumulating plants include Halogeton, Sarcobatus,
Rheum, Oxalis, and Rumex species, as well as some cultivated species such
as mangels and sugar beets. The fungi Aspergillus niger and A. flavus can
produce large quantities of oxalates on feedstuffs, and large doses of ascorbic
acid have caused oxalate nephrotoxicosis in humans and in a goat.
In monogastric animals, ingestion of ethylene glycol (EG) is the most
common source of renal oxalosis. Metabolites of EG play the major role in
pathophysiology. In the liver, ethylene glycol is metabolized to glycoaldehyde,
which depresses respiration and serotonin metabolism and alters CNS amine
concentrations. Glycoaldehyde is rapidly metabolized to glycolate, which
causes severe metabolic acidosis, and is thought to be the primary toxic
metabolite of EG. Glycolate is oxidized to glyoxalate, which is the most
toxic metabolite on a per- weight basis; however, its half-life is very
short. Glyoxalate inhibits citric acid cycle enzymes and substrate level
phosphorylation in mitochondria. Glyoxalate is oxidized to oxalate, which
binds with calcium and forms a soluble complex which is filtered by glomeruli.
As water is reabsorbed by the tubules and the pH of the filtrate decreases,
calcium oxalate precipates to form crystals. This results in nephrosis,
and hypocalcemia if enough calcium is complexed. The cause of death is hyperkalemia
resulting in cardiac arrest.
Some of the conference participants observed an increased tortuosity
of interlobular arteries in this case. There was discussion concerning whether
this finding was due to an overall increase in the length of these vessels
or was a result of the marked interstitial fibrosis and cortical loss.
Contributor: C.E. Kord Animal Disease Laboratory, P.O. Box 40627,
Melrose Station, Nashville, TN 37204
References:
- 1. Rhyan JC, et al: Severe renal oxalosis in 5 young Beefmaster calves.
JAVMA 201 (12): 1907-1910, 1992.
2. Gopal T, et al: Renal oxalosis in neonatal calves. Vet. Pathol: 15,519-524,1978.
- 3. Maxie MG: The urinary system. In: Pathology of Domestic Animals,
4th ed., Jubb, Kennedy, and Palmer, eds. Academic Press, Inc. pp.491-3;
1993.
International Veterinary Pathology Slide Bank:
Laser disc frame #102, 9561,9562.
Case III - 97BKB9 (AFIP 2594277)
Signalment: 8 to 10-year-old Holstein cow.
History: This cow was from a large dairy herd in Texas. The herd
was depopulated due to large number of animals that tested positive for
the tuberculosis intradermal skin test. This animal was transported to the
National Animal Disease Center to participate in an ongoing research program.
Gross Pathology: There was a roundish, 10 cm diameter firm, white-
yellow, multilobular mass adjacent to the proximal duodenum and cranial
to the right kidney. The mass was estimated to weigh 2-3 lbs. In addition,
there were multiple fibrous adhesions of the pleura to the chest-wall, multiple
tubercles in lung parenchyma, and massive enlargement of mediastinal lymph
nodes. The cut surface of lung and lymph node lesions revealed abundant
caseous exudate centrally surrounded by a thick fibrous capsule.
Laboratory Results: Large numbers of Mycobacterium bovis were
isolated from lymph node and lung.
Contributor's Diagnosis and Comments:
Adrenal gland: pheochromocytoma.
Section of adrenal gland containing a 1-2 x 2-3 cm neoplastic mass bordered
on one side by compressed adrenal cortex covered by a thick fibrous capsule
and otherwise extending to the edges of the section. The neoplastic mass
is arranged in an alternating pattern of loosely assembled and more densely
grouped cells. Cells are arranged in lobules, elongate packets, cords and
in perivascular palisades of one to four cell layers. Lobules have central
variably-sized spaces containing amorphous eosinophilic material admixed
with amphophilic to basophilic fibrils, or they contain pyknotic cells or
mineralized cell debris. The cell groupings are separated by a delicate
fibrovascular stroma. Neoplastic cells are large (15 - 25 µm), polyhedral,
have indistinct borders and abundant granular amphophilic cytoplasm. Nuclei
are 10-20 µm, round to oval, centrally located, and contain finely-dispersed
granular chromatin and 1 to 3 conspicuous nucleoli. Moderate numbers of
cells with pyknotic nuclei are interspersed throughout the mass. The mitotic
index is low ( less than 2/ Hpf).
Small groupings of non-neoplastic medullary cells arranged in nests and
cords are separated by thick parallel bands of moderately vascularized fibrous
tissue. In a few areas, cords of neoplastic cells from the underlying mass
extend into and appear contiguous with medullary tissues.
Pheochromocytomas are the most common tumors of the adrenal medulla of
animals. They occur most often in dogs and cattle, have also been reported
in the horse, cat, and rat, but overall are infrequent neoplasms of animals.
They are often incidental findings at necropsy. Functional pheochromocytomas
can produce signs related to excessive catecholamine secretion such as tachycardia
and dependent edema. Metastases are rare, except in dogs, but pheochromocytomas
have a tendency to invade local tissues, especially the caudal vena cava,
where neoplastic emboli may be seen.
The pheochromocytoma was considered to be an incidental finding in this
case and not related to the primary disease caused by M. bovis infection.
Differential diagnosis for gross lesions includes adrenal cortical adenoma/
carcinoma, neuroblastoma, and lymphoma.

Case 3. Adrenal gland. Polygonal cells are infiltrating tumor capsule.
(20x obj)
AFIP Diagnosis:
Adrenal gland: Adrenocortical carcinoma, Holstein, bovine.
Conference Note: Although careful consideration was given to the
contributor's diagnosis, a majority of the conference participants favored
adrenocortical carcinoma. Distinguishing between pheochromocytomas and adrenocortical
carcinomas is sometimes difficult. Features which were considered to be
significant in the diagnosis of this case included an absence of fine granulation
in the neoplastic cells, multifocal mineralization, and scattered foci of
necrosis. A Cherukian-Schenk (argyrophilic) stain performed at the AFIP
was non-contributory due to non-specific staining. Likewise, immunohistochemical
stains for synaptophysin and neuron specific enolase were not helpful due
to lack of staining of internal positive controls. This case was reviewed
by the AFIP's Division of Endocrine Pathology, whose pathologists unanimously
agreed that the tumor is more likely an adrenocortical carcinoma than a
pheochromocytoma, though the pattern differs from what is usually seen in
human cases. They would also like to exclude the possibility of metastasis
from another site.
Adrenal cortical carcinomas have been reported most often in cattle,
dogs, and ferrets. They are usually larger than cortical adenomas and, in
dogs, are more likely to be bilateral. In cattle, bilateral involvement
is seldom observed, and metastasis is rare. In sheep, up to 40 percent of
cortical carcinomas metastasize to other organs. Often, they invade extensively
into surrounding tissues, including the caudal vena cava. Carcinomas may
attain considerable size in cattle (up to 25 cm). Multiple areas of mineralization
or ossification, hemorrhage, and necrosis are common findings in adrenocortical
tumors of cattle. Functional tumors are associated with atrophy of the contralateral
adrenal cortex via negative feedback inhibition of pituitary ACTH secretion.
Contributor: USDA/ARS/National Animal Disease Center, 2300 Dayton
Avenue, Ames, IA 50010
- References:
1. Buckingham JD. Pheochromocytoma in a mare. Can. Vet. J. 11:205-208,
1970.
- 2. Cheng L. Pheochromocytomas in rats: Incidence, etiology, morphology,
and functional activity. J. Environ. Pathol. Toxicol. 4:219-228, 1980.
- 3. Maher ER, McNeil EA. Pheochromocytoma in dogs and cats. Vet. Clin.
N. Am. 27: 359-380, 1997.
- 4. West JL. Bovine pheochromocytoma: Case report and review of literature.
Am. J. Vet. Res. 36: 1371-1373, 1975.
- 5. White RA, Cheyne IA. Bone metastases from a phaeochromocytoma in
the dog. J. small Anim. Pract. 18: 579-584, 1977.
- 6. Capen CC. The endocrine glands. In: Pathology of Domestic Animals,
4th ed., Jubb, Kennedy, and Palmer, eds. Academic Press, Inc. Vol. 3, p.
337. 1993.
- 7. Monlux WS, Monlux AW. Atlas of Meat Inspection Pathology. United
States Department of Agriculture, Agriculture Handbook No. 367, pp. 24-25,
1972.
-
International Veterinary Pathology Slide Bank:
Laser disc frame #8215, 10693, 14784, 23782
Case IV - 97:1144 (AFIP 2594508)
Signalment: 7-year-old male Bernese Mountain Dog.
History: The dog developed sudden unilateral hyphema and glaucoma.
Ophthalmic examination identified a white mass in the ventral anterior chamber.
The eye was enucleated and submitted for biopsy.
Gross Pathology: The globe was symmetrically enlarged. An oval,
well- demarcated 2-3 mm ulceration of the central cornea was present. On
section, the iris and anterior segments of the uvea were widened, firm,
and dark brown to black.
Laboratory Results: None available.
Contributor's Diagnoses and Comments:
- 1. Malignant Histiocytoma; eye
2. Inner retinal atrophy, diffuse, moderate
- 3. Corneal edema and neovascularization, multifocal, moderate
The normal anatomy of the filtration angle, iris, and ciliary body is
effaced and expanded by an unencapsulated, infiltrative, cellular mass with
lateral borders that extend through the width of the sclera. The mass consists
of densely packed sheets and aggregates of pleomorphic round cells. Individual
cells are large with abundant eosinophilic, often vacuolated cytoplasm,
and generally distinct cytoplasmic borders. Nuclei are variable in shape
and size with coarsely clumped or marginated chromatin and one to multiple
nucleoli. Mitotic figures average 2-7 per high power field and often have
bizarre morphology. Interspersed among these cells are multinucleated giant
cells. There is phagocytosis of cellular debris and inflammatory cells by
mononuclear and multinucleated cells. The cellular morphology of large pleomorphic
mononuclear cells and multinucleated giant cells with abundant often vacuolated
cytoplasm, as well as the observation that these cells are actively phagocytic
suggests a histiocytic origin. Changes in the cornea and retina are considered
secondary to glaucoma due to neoplastic obliteration of the filtration angle.
An additional biopsy of a subcutaneous mass from this animal was composed
of neoplastic cells of similar morphology to those infiltrating the eye.
Malignant histiocytosis is a familial disease of Bernese Mountain Dogs
most predominantly affecting aging male dogs (mean age of onset 7 years).
The disorder has a rapidly progressive clinical course and is characterized
by proliferation of atypical histiocytic cells within multiple organ systems.
Pulmonary, splenic, and hepatic involvement occurs with high frequency.
The
eye and skin are rarely affected. In case studies, ultrastructural morphology
and immunohistochemical demonstration of lysozyme and alpha-1-antitrypsin
in the proliferating cells is consistent with a macrophage origin.
Malignant histiocytosis is clinically and pathologically distinct from
systemic histiocytosis, which also has a familial occurrence in the Bernese
Mountain Dog. Systemic histiocytosis occurs in younger dogs (mean age of
onset 4 years) and has a prolonged fluctuating clinical course. The skin
and peripheral lymph nodes are commonly affected. In this disorder, infiltrating
histiocytic cells lack cellular atypia and multinucleated giant cells are
rare.

Case 4. Pleomorphic histiocytes & histiocytic giant cells are invading
scleral collagen. (10x obj).
AFIP Diagnoses:
- 1. Eye: Malignant histiocytosis, Bernese Mountain Dog, canine.
- 2. Eye, cornea: Neovascularization, multifocal, mild.
- 3. Eye, retina, ganglion cell layer: Degeneration and loss, multifocal,
mild.
- 4. Eye, globe: Dilatation, diffuse, mild, with scleral atrophy.
Conference Note: Conference participants agreed with the contributor's
comments. Malignant melanoma and malignant histiocytosis were considered
in the differential diagnosis. Malignant histiocytosis was favored due to
a variety of factors, including evidence of phagocytosis by the neoplastic
cells, the presence of many multinucleated cells, and the breed of the dog.
Contributor: University of Connecticut, Department of Pathobiology,
U- 89, 61 North Eagleville, Storrs, CT 06269-3089.
- References:
1. Moore PF, Rosin A : Malignant histiocytosis of Bernese Mountain Dogs.
Vet. Path 23:1-10, 1986.
- 2. Rosin A, Moore PF, Dubielzig R: Malignant histiocytosis in Bernese
Mountain Dogs. JAVMA vol 188, 9:1041-1045 1986.
- 3. Ramsey JK, McKay JS, Rudorf H, Dobson JM: Malignant histiocytosis
in three Bernese Mountain Dogs. Vet Rec 138:440-444, 1996.
- 4. Scherlie PH, Smedes SL, Feltz T, Douherty SA, Riis RC : Ocular manifestation
of systemic histiocytosis in a dog. JAVMA vol 201(8):1229-1232, 1992.
International Veterinary Pathology Slide Bank:
Laser disc frame: None.
Terrell W. Blanchard
Major, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: blanchard@email.afip.osd.mil
* The American Veterinary Medical Association and the American College
of Veterinary Pathologists are co-sponsors of the Registry of Veterinary
Pathology. The C.L. Davis Foundation also provides substantial support for
the Registry.
Return to WSC Case Menu.