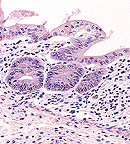
 Mild lymphoplasmacytic typhitis
in a 10-week-old mouse fed DSS for 5 days. (40X, HE, 78K)
Mild lymphoplasmacytic typhitis
in a 10-week-old mouse fed DSS for 5 days. (40X, HE, 78K)
 Mild lymphoplasmacytic typhitis
in a 10-week-old mouse fed DSS for 5 days. (40X, HE, 78K)
Mild lymphoplasmacytic typhitis
in a 10-week-old mouse fed DSS for 5 days. (40X, HE, 78K)
Signalment: 10-week-old male mouse (Mus musculus) of the (C3H/HeJBir x C57BL/6J)F1 x C57BL/6J backcross.
History: To induce inflammatory bowel disease, this mouse was fed 5% dextran sulfate sodium (DSS) in the water for a period of 5 days. During this time, clinical features included occult blood in the feces, soft stool, and loss of body weight. After an additional 5 days on water without DSS, the mouse was necropsied. The intestinum was removed and a "Swiss roll" was prepared for histopathological evaluation. The cecum was inflated with fixative, bisected, and processed.
Gross Pathology: The mouse had a small, shrunken, yellow cecum that contained little ingesta. The colon was slightly distended with mustard yellow colored feces that formed soft oval pellets. This soft fecal material became matted in the perianal hairs.
Laboratory Results: Microbiological assays were negative for known mouse pathogens.
Contributor's Diagnosis and Comments: Severe, chronic, active, ulcerative typhlitis. Etiology: Dextran sulfate sodium induced ulcerative colitis.
C3H/H3JBir is a new substrain of C3H/HeJ mice with a high incidence of spontaneous colitis. The disease is clinically manifested by occult blood, soft yellow feces and perianal ulceration. Microscopically, lesions are primarily limited to the cecum and right colon and characterized by acute and chronic inflammation, crypt abscesses, ulcerations, regenerative hyperplasia, and submucosal scarring. The disease does not appear to be progressive or recurrent and older mice are rarely affected. Up to now, all diagnostic tests have failed to identify any known mouse pathogens. It is concluded that C3H/HeJBir mice have a genetic background that increases susceptibility to a spontaneous form of murine inflammatory bowel disease (IBD). These findings resemble those of IBD in man and suggest C3H/HeJBir mice as an animal model of IBD. In addition, C3H/HeJBir mice have been found to be highly susceptible to experimental murine colitis induced by dextran sulfate sodium (DSS). Some of the DSS susceptibility genes may be identical to those responsible for the increased incidence of spontaneous colitis in C3H/HeJBir mice. The goal of the current study is to determine the number and chromosomal location of DSS susceptibility genes in this strain. This information will predict the location of homologous genes in the human genome using the comparative map for mice and humans.
AFIP Diagnosis: Cecum and colon: Enteritis, lymphoplasmacytic and neutrophilic, erosive, multifocal, moderate, CH3/HeJBir mouse, rodent.
Conference Note: Crohn's disease and ulcerative colitis are idiopathic intestinal diseases of humans that together are referred to as inflammatory bowel disease (IBD). There appears to be a genetic predisposition for development of IBD in humans. Similar diseases with genetic predispositions occur in animals, namely histiocytic ulcerative colitis of boxers, idiopathic mucosal (lymphoplasmacytic) colitis of dogs and cats, and colitis of cotton-topped tamarins.
Crohn's disease is very similar to histiocytic ulcerative colitis of Boxers. Crohn's disease is characterized by transmural granulomatous inflammation of the small intestine and colon; affected segments are sharply delineated from adjacent normal tissue. Mycobacterium paratuberculosis has been implicated as a possible cause of Crohn's disease. Crohn's disease is associated with polyarthritis, sacro- iliitis, ankylosing spondylitis, uveitis, cholangitis, and skin lesions.
Ulcerative colitis is a ulcerative inflammatory disease that is confined to the mucosa of the colon and bears many similarities to idiopathic mucosal colitis of dogs and cats. Lesions extend in a continuous fashion from the rectum. Ulcerative colitis is associated with extraintestinal lesions similar to those listed for Crohn's disease.
This proposed murine model of IBD may allow investigation into the early pathophysiology of intestinal lesions associated with IBD, as well as the study of the genetic factors which predispose individuals to IBD.
Differential diagnosis for intestinal inflammation and ulceration in mice includes mouse hepatitis virus, epizootic diarrhea of infant mice, salmonellosis, clostridial infection, and E. coli infection.
Contributor: The Jackson Laboratory, 600 Main Street, Bar Harbor, ME 04609-1500.
References:
 Liver from a Syrian hamsters.
Note the accentuated lobular pattern. (17K)
Liver from a Syrian hamsters.
Note the accentuated lobular pattern. (17K)
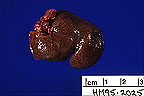
 Swollen, pale, pitted kidneys
from a Syrian hamster. (17K)
Swollen, pale, pitted kidneys
from a Syrian hamster. (17K)
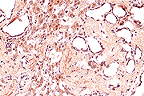 Hepatic amyloidosis in a Syrian
hamster (40X, Congo Red, 88K)
Hepatic amyloidosis in a Syrian
hamster (40X, Congo Red, 88K)
Signalment: 1-year-old female Syrian hamster
History: This was a sentinel animal, that was noted to have ocular discharge from the right eye. She was submitted for a total health status work-up.
Gross Pathology: The hamster was severely dehydrated, with moderate body fat stores. She had scant ocular discharge from both eyes. The liver had an accentuated lobular pattern with pale centrilobular zones. Both kidneys were diffusely pale, firm, with a granular pitted cortical surface. The left atrium was distended with a chronic thrombus.
Laboratory Results: Urinalysis (multistix strip applied against the mucosa of the empty urinary bladder): Ketones = 80 mg/dL, specific gravity = 1.030. Bacterial culture of conjunctival sac recovered Staphylococcus hyicus and Streptococcus suis.
Contributor's Diagnosis and Comments: Liver: Amyloidosis, diffuse, moderate, portal and periportal, Syrian Hamster. Etiology: Secondary amyloidosis.
Amyloidosis is an important cause of morbidity and mortality in Syrian hamsters. It's incidence is highest in female hamsters older than 18 months. The incidence is apparently genetically influenced, with marked variations recorded among different colonies. Liver, kidneys, spleen, and adrenal glands are the organs most often involved. In the liver, amyloid deposits first within vascular walls and portal areas. With time, amyloid fills the portal area and fingers out into the adjacent parenchyma, causing accentuation of the lobular pattern which can be seen grossly. In the kidneys, amyloid deposits primarily within glomeruli, along basement membranes and within mesangium.
Hamster female protein (FP), a sex-limited protein which in Syrian females is found in the serum at 100 fold increased concentration over that of intact males (with castrated males having intermediate levels), is likely one factor to explain the increased incidence of amyloidosis in females. FP is structurally homologous with human C reactive protein, and hamster amyloid deposits are enriched with FP. Hamster amyloid fibrils are composed of AA protein (derived from serum amyloid A which is induced by the variety of inflammatory cytokines) and it is believed that FP may promote fibrillogenesis and/or stabilize and protect fibrils once they are formed.
Diet can also influence the incidence of amyloidosis in Syrian hamsters. Female Syrian hamsters fed a semi-purified diet had increased longevity and decreased incidence of amyloidosis compared to those fed a commercial diet. Consumption of a high protein diet is also associated with increased incidence of amyloidosis.
Amyloidosis of Syrian hamsters is considered a valuable model of human secondary amyloidosis.
AFIP Diagnosis: Liver: Amyloidosis, portal and centrilobular, bridging, diffuse, moderate, with bile duct hyperplasia, Syrian hamster, rodent.
Conference Note: Amyloid is a protein substance that accumulates in the extracellular spaces of many organs in association with different disease processes. Ultrastructurally amyloid is made up of fibrils of indefinite length with a diameter of 7.5 to 10 nm. The fibrils form a beta-pleated sheet (this conformation confers birefringence under polarized light). There is always a second component, the P component, present within the amyloid; ultrastructurally it appears as a pentagonal doughnut-shaped structure. Fifteen different types of amyloid protein have been identified, of these two protein types are more common: amyloid light chain (AL) protein and amyloid-associated (AA) protein.
Amyloid light chain protein is formed from immunoglobulin light chains (predominantly lambda light chains) and is associated with plasma cell dyscrasia. When amyloidosis occurs in conjunction with immunocyte dyscrasia it is known as primary amyloidosis.
Amyloid-associated protein is associated with chronic inflammation, and causes what is known as secondary amyloidosis. Amyloid-associated protein is derived from serum amyloid-associated protein (SAA). SAA synthesis in the liver is increased under the influence of IL-1 and IL-6. Elevation of serum SAA is not sufficient to induce systemic amyloidosis. It has been proposed that individuals with amyloidosis may be deficient in the enzyme response for the reduction of SAA or AA, or individuals may form altered AA or SAA protein that are resistant to degradation.
Serum amyloid P component (SAP) is the precursor for amyloid P component. The hamster female protein is homologous to SAP as well as C reactive protein; SAP and C reactive proteins are acute phase reactants. There is a strong association between SAP, hamster FP and the formation of amyloid. It is hypothesized that FP is reduced to a homologue of the P component and stimulates fibrillization of AA or prevents degradation of AA by stabilizing existing fibrils. The similarity of SAP and FP may allow study of amyloid P component and secondary amyloidosis.
Many Syrian hamsters develop systemic amyloidosis; the liver, spleen, and kidneys are commonly affected. Hamsters with systemic amyloidosis often develop severe glomerular amyloidosis. The damaged glomeruli leak large amounts of protein causing hypoproteinemia which results in ascites, dependant edema, and hydrothorax.
Contributor: National Institutes of Health, NCRR/VRP, 28 Library Drive MSC 5230, Bethesda, Maryland 20892-5230.
References:
International Veterinary Pathology Slide Bank:
Laser disc frame #585, 1187-8, 2574, 2947, 3037-9, 4544-6, 4697,
4736, 5400, 9218, 9253, 9282, 21180-2, and 24165.
Case III - 95B5056 (AFIP 2503158)
Signalment: 1-year-old Angus bull.
History: A bull in a sheep and cattle feedlot operation developed fever (107 degrees F), weakness, anorexia, mucopurulent nasal discharge, and keratitis. After four weeks of illness, the bull's condition began to improve. He remained blind due to bilateral pigmentary keratitis. The animal was killed and examined 76 days after onset of clinical signs.
Gross Pathology: The carcass was in fair-to-good condition (382 kg). Six small (2-5 mm diameter) ulcers were on the lateral margins of the tongue. There was heavy pigmentation of the corneal epithelium due to radial striae that extended from the pigmented limbus. Four thick-walled abscesses up to 4 cm diameter were in the liver.
Vascular abnormalities were not evident grossly.
Laboratory Results: Peripheral blood leukocytes were positive by polymerase chain reaction (PCR) assay for a 239-bp DNA fragment of an ovine gammaherpesvirus, ovine herpesvirus-2 (OHV-2). The bull was also positive serologically by competitive inhibition ELISA for the same, or a closely related, gammaherpesvirus. Virus isolation was attempted on peripheral blood leukocytes and was negative. No bacteria were grown from the hepatic abscesses.
.
Contributor's Diagnosis and Comments: Arteriosclerosis, moderate, multifocal, chronic, hyperplastic, with mild multifocal lymphocytic arteritis, pampiniform plexus
Etiology: Malignant catarrhal fever.
Similar vascular lesions were found in carotid rete, meninges, upper respiratory tract mucosa, kidney and eyelids. Other lesions were moderate multifocal lymphocytic uveitis-retinitis, chronic stromal keratitis, localized lymphocytic myocarditis with fibrosis, mild diffuse nonsuppurative encephalomyelitis, multifocal necrotizing glossitis-esophagitis, and severe diffuse atrophy of seminiferous tubules. No infarctive lesions were found.
Malignant catarrhal fever is generally associated with a high case fatality rate (>95%). In the past 3 years, this laboratory has seen 5 cases of MCF from 3 premises in which cattle with acute MCF recovered. Some cattle recovered sufficient body weight to go for slaughter and their carcasses were passed for human consumption. The characteristic lesion in such cattle is disseminated obliterative arteriopathy. The hyperplastic cells in the arterial intima are of smooth muscle origin, on the basis of ultrastructural and immunocytochemical studies. Typical features in the vasculopathy include disruption of the inner elastic lamina, and thinning and neovascularization of the tunica media. Veins are unaffected. Arteriosclerosis is presumably a sequel to the characteristically florid lymphocytic arteritis of acute MCF.
Sheep-associated MCF is attributed to OHV-2, which is referred to by some as malignant catarrhal fever virus (MCFV). Unlike the wildebeest-associated MCF agent (alcelaphine herpesvirus-1; AHV-1) which has been isolated, the sheep- associated agent has never been grown in culture. Molecular techniques do however permit recognition of DNA sequences unique to OHV-2 in blood and tissue. All 5 animals that recovered from MCF and were examined by this laboratory, including this one, remained persistently infected with OHV-2 according to the PCR assay. The site of viral persistence in tissues remains to be established.
AFIP Diagnosis: Spermatic cord, arteries, area of pampiniform plexus (per contributor): Intimal proliferation, multifocal, moderate, with medial disruption and lymphoplasmacytic and neutrophilic arteritis, Angus, bovine.
Conference Note: Malignant catarrhal fever (MCF) is usually an acute disease characterized by lymphocytic arteritis and necrosis of small and medium sized arteries. However, in cattle that survive acute MCF the arterial lesions are proliferative, characterized by intimal proliferation and disruption of the tunica media and the internal elastic lamina.
Similar proliferative arterial lesions can be seen in conditions that induce endothelial damage such as dirofilariasis and elaeophoriasis, pulmonary hypertension, balloon-catheter de-endothelialization and graft associated arteriosclerosis in transplanted organs. Viruses other than MCFV have been implicated in arteriopathies; avian herpes virus type 2 in chickens (Marek's's disease), simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) have been associated with arterial intimal proliferation, smooth muscle hyperplasia and segmental sclerosis. Possible relationships between viral infection and development of human atherosclerosis are under investigation.
Contributor: Wyoming State Veterinary Laboratory, 1174 Snowy Range Road, Laramie, WY 82070.
References:
International Veterinary Pathology Slide Bank:
Laser disc frame #4997-9, 5677, 9357, 18604, 19541, 19793, 20189,
and 21981-5.
Signalment: 15-year-old male Chow Chow.
History: The dog presented with loss of bodyweight and frequent vomiting, irrespective of food intake. Clinical pathology results were unremarkable. Laparotomy revealed a 5 cm gastric mass which was surgically excised. The dog was euthanized 8 days later due to deteriorating condition. Necropsy was not permitted by the owner.
Gross Pathology: The surgical specimen was a section of stomach containing a 5 cm diameter homogeneous nodule in its wall, raising both the serosal and mucosal aspects. The mucosa was focally ulcerated.
Laboratory Results: Not applicable.
Contributor's Diagnosis and Comments: Stomach: Plasma cell tumor with amyloid.
The tumor lies in the submucosa, infiltrating the lamina propria focally, is poorly demarcated, non-encapsulated and subdivided into large nests by a fine fibro-vascular stroma. Multifocally there is a moderate infiltrate of primarily lymphocytes or hemosiderin-laden macrophages.
Neoplastic cells are pleomorphic, have an abundant eosinophilic to amphophilic cytoplasm and contain one to three variably sized and shaped nuclei, occasionally more. The chromatin is stippled to peripheralized and a large nucleolus is often seen, sometimes giving the characteristic clock-faced pattern.
Among the morphological characteristics, the most conspicuous ones are the cellular and nuclear pleomorphism, the abundant homogeneous cytoplasm and the presence of amyloid. The amyloid appears as large aggregates scattered in the stroma and blood vessel walls, within and close to the tumor. Varying numbers of macrophages and foreign body type giant cells are consistently associated with amyloid deposits.
The stomach is one of the reported sites for this tumor, together with the rectum, colon, intestine, spleen, and oral cavity. The site of predilection is the skin, especially lip, ear, and limbs. Neoplastic cells typically show positivity for vimentin and IgG on immunohistochemistry.
The prognosis of this tumor of older dogs is good, most (more than 90%) being cured by complete surgical excision and recurrence at the site is rare. However, malignant variants cannot be identified on the basis of infiltrative or cytologic characteristics. A combination of DNA aneuploidy and c-myc content analysis has been shown to be of some value in establishing a more accurate prognosis.
Amyloid deposition is found in a small proportion of cases and its presence does not correlate with the behavior. It has been established in a number of these neoplasms that the amyloid is composed of light chain immunoglobulins but the mechanism of deposition remains to be established. Systemic amyloidosis is not found. In this case, the amyloid stained weakly positive with antibodies for IgA, and negative for IgG.
Extramedullary plasma cell tumors must be distinguished from multiple myeloma. This will be achieved by a thorough clinical evaluation looking for osteolytic lesions, paraproteinemia and Bence Jones proteinuria.
AFIP Diagnosis: Stomach: Plasma cell tumor with amyloid, Chow Chow, canine.
Conference Note: Ultrastructural characteristics of plasma cell tumors include abundant rough endoplasmic reticulum and prominent Golgi. The Golgi is responsible for the perinuclear hof that is visible by light microscopy.
The differential diagnosis for plasmacytoma includes mast cell tumor, carcinoid, and lymphosarcoma. In this case, the nest-like pattern with fine fibrovascular stroma and cellular morphology (round cells with abundant eosinophilic cytoplasm, perinuclear hofs, eccentric nuclei that are round or crescentic, the presence of marked anisocytosis and anisokaryosis, uninucleate giant neoplastic cells, and multinucleate neoplastic cells) are highly characteristic of plasma cell tumor.
Contributor: Laboratoire Pfizer, Centre de Recherche, BO 159, Ariboise cedex, France, 37401.
References:
International Veterinary Pathology Slide Bank:
Laser disc frame #1375-6, 8344, and 21493-5.
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.