Signalment:
Gross Description:
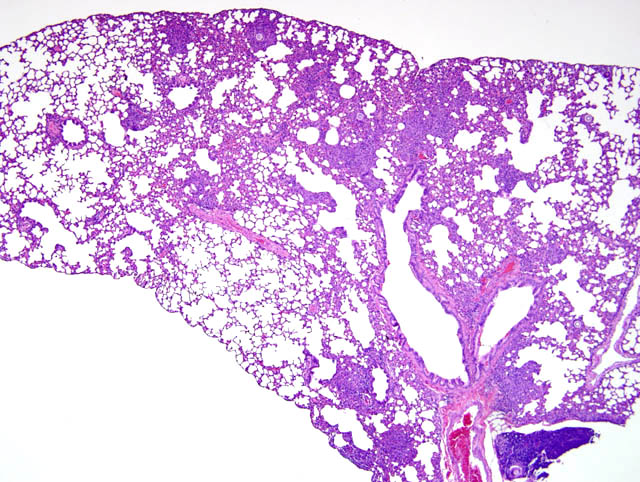
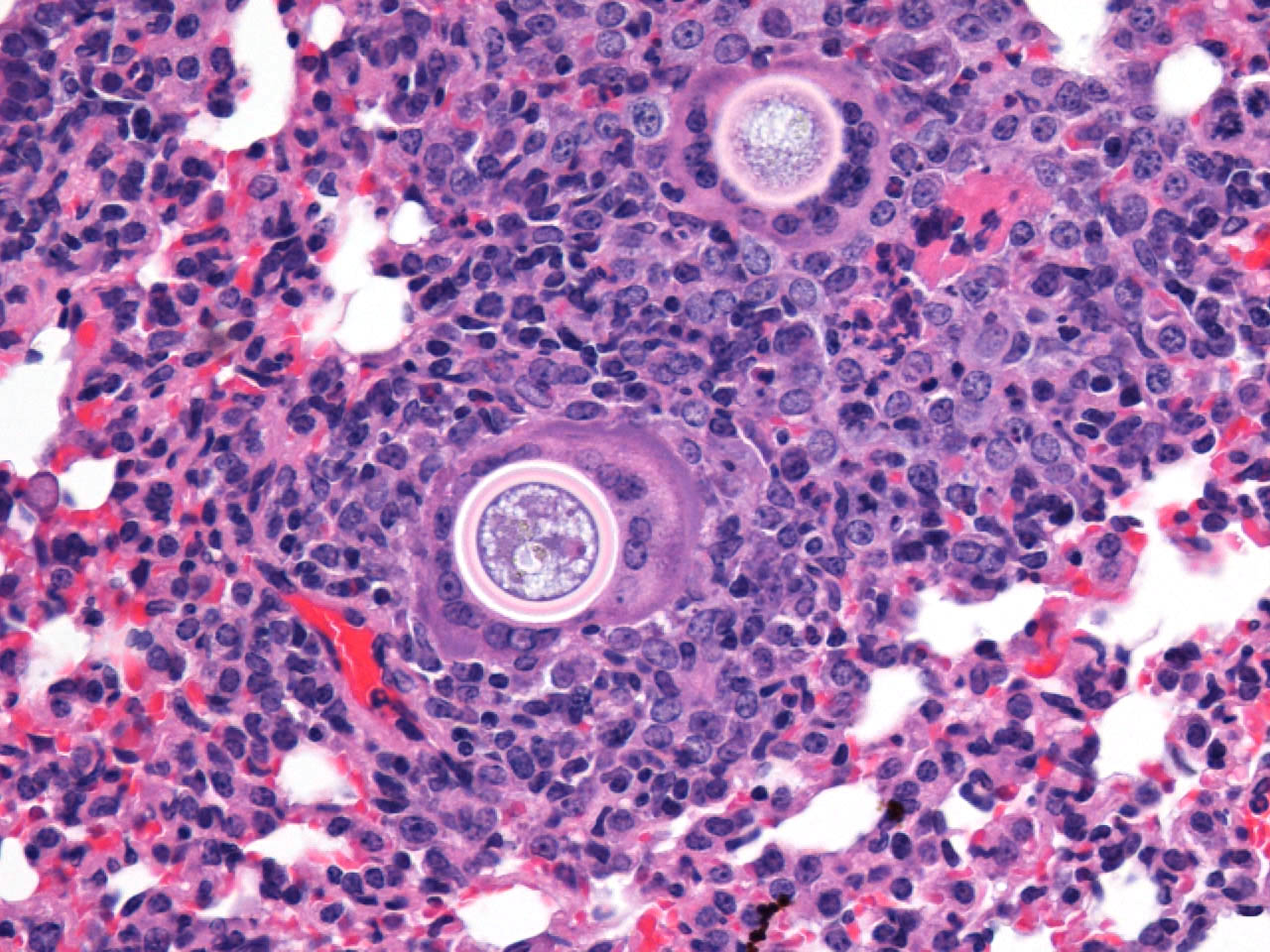
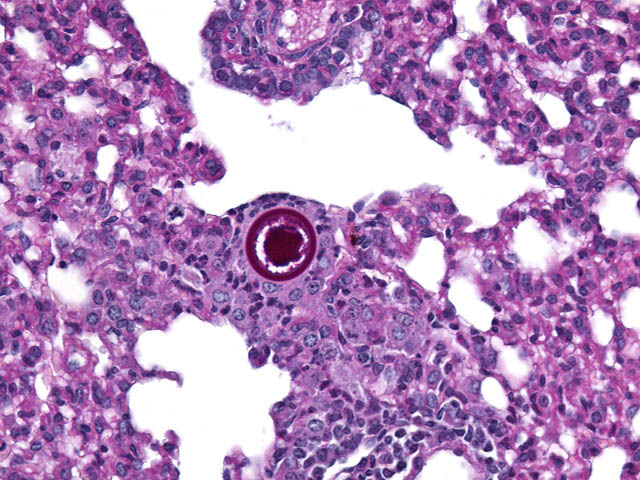
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Two varieties of the fungal genus Chrysosporium, formerly known as Emmonsia, cause adiaspiromyosis: C. parvum var. parvum and C. parvum var. crescens. The latter has the widest host range and distribution. 1 C. parvum var. crescens is a dimorphic fungus with spores that are 2-4μm in the environment. They enlarge to 200-400μm when inhaled and incubated at body temperature. At lower temperature (20-30oC) the spores will develop into a mycelial form.3
The typical pathologic feature of the infection is the granuloma or pyogranuloma which appear grossly as gray white nodules in the lungs.1 The lung is the only organ known to be infected.4 Histologically, these nodules contain a central adiaspore surrounded by varying degrees granulomatous to pyogranulomatous inflammation. Ruptured spores incite the most severe reactions.1 Adiaspores range from 200-400μm in diameter for C. parvum var. crescens and 20-40μm C. parvum var. parvum.2 C. parvum var. crescens has a characteristic thick cell wall that is up to 70μm thick.3 The center of the adiaspore contains a mass of amphophilic to basophilic small globules.2 There is a single nucleus in spores of C. parvum var. parvum, however C. parvum var. crescens develops multiple nuclei as it enlarges.2 There is no budding or endosporulation.1
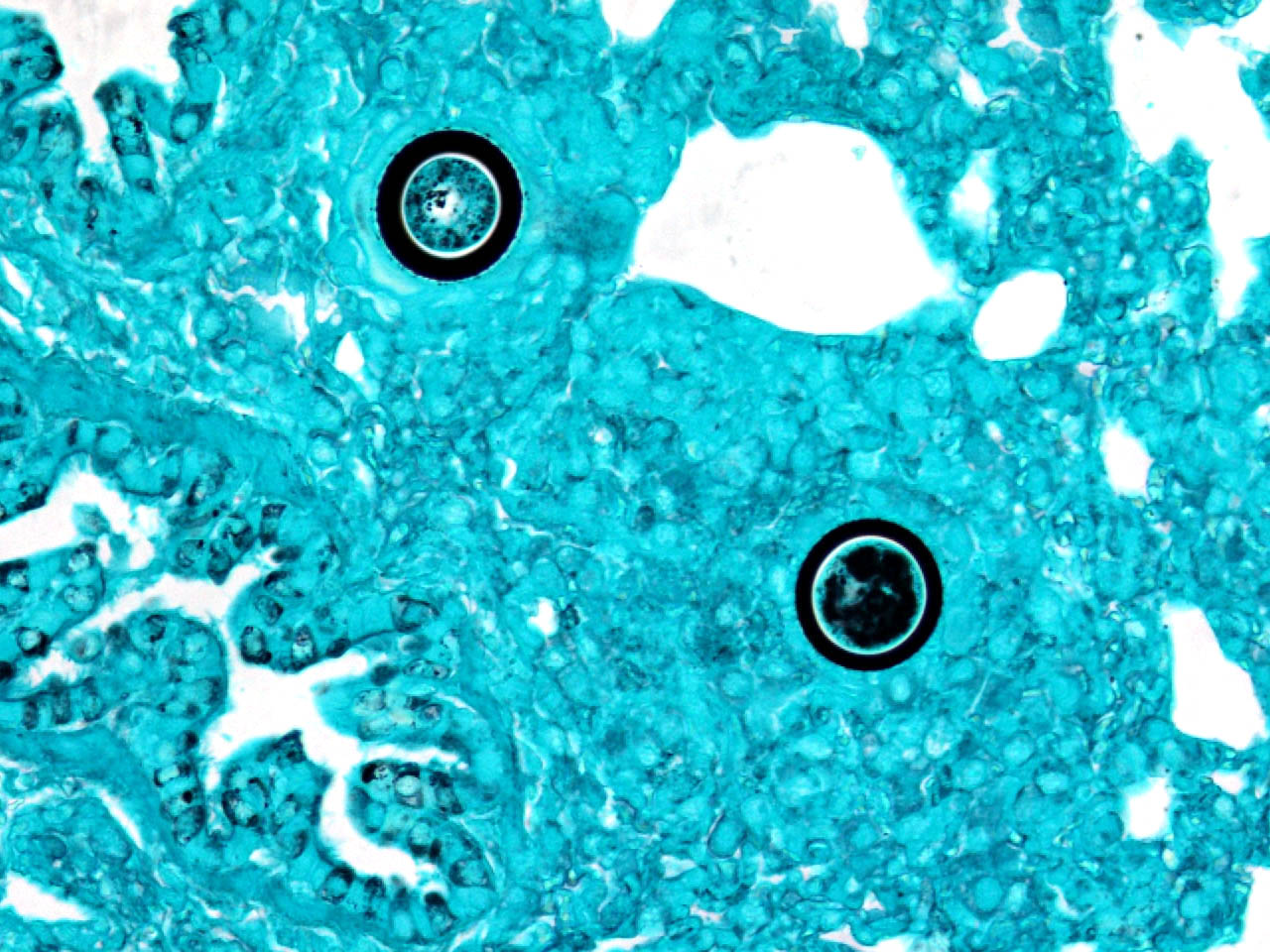
Differential diagnosis includes other fungi of similar size and morphology, such as Coccidioides immitis and Rhinosporidium seeberi. Morphologically, C. parvum (20-70μm) has a thick capsule while C. immitis (12μm) and R. seeberi (35μm) have relatively thin capsules.3 The presence of endospores occurs with C.immitis and R. seeberi, but not C. parvum.3 C. parvum infection does not produce hyphae, unlike C. immitis. 3 Histochemically, the capsules of all three stain with PAS and GMS.3 The capsule of R. seeberi also stains with mucicarmine, unlike the other two.3
The infection has been reported in mice, moles, rats, rabbits, ground squirrels, weasels, martens, minks, armadillos, wallabies, skunks, opossums, dogs, cats, raccoons, and humans.4
The disease in immunocompetent animals (including humans) is typically benign, self-limiting, and confined to the lungs. However, clinical signs can occur with heavy infections.1 Most infections are considered incidental findings during the course of histopathologic evaluation of the lungs as in these ground squirrels.Â
Of the 20 ground squirrels examined during this study, adiaspiromycosis was detected in 13 of them (60.5%). Interestingly, only 1 of 18 (5.1%) prairie dogs in this study was infected. The reasons that two species of burrowing rodents from the same geographical area had such different prevalences of infection were not determined.Â
JPC Diagnosis:
2. Kidney: Essentially normal tissue.
Conference Comment:
Inhaled dust-borne aleurioconidia (2-4 um) do not germinate in the host, but instead dramatically enlarge into thick-walled adiaspores. Clinically, infection may range from asymptomatic to severe necrogranulomatous pneumonia depending on adiaspore load and immunocompetence of the host.5 Infection is not considered transmissible between individuals.7,8
References:
2. Caswell JL, Williams KJ: Respiratory system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, p. 645. Elsevier Limited, St. Louis, MO, 2007
3. Chandler FW, Kalpan W, Ajello L: Adiaspiromycosis. In: Color Atlas and Text of the Histopathology of Mycotic Diseases, pp. 3033. Year Book Medical Publishers, Chicago, IL, 1980
4. Chandler FW, Watts JC: Adiaspiromycosis. In: Pathologic Diagnosis of Fungal Infections, pp. 3541. ASCP Press, Chicago, IL, 1987
5. Chantrey JC, Borman AM, Johnson EM, Kipar A: Emmonsia crescens infection in a British water vole (Arvicola terrestris). Med Mycol 44:375-378, 2006
6. Hamir AN: Pulmonary adiaspiromycosis in raccoons (Procyon lotor) from Oregon. J Vet Diagn Invest 11:565567, 1999
7. Hub+�-�lek Z, Burda H, Scharff A, Heth G, Nevo E, _umbera R, Pe_ko J, Zima J: Emmonsiosis of subterranean rodents (Bathyergidae, Spalacidae) in Africa and Israel. Med Mycol 43:691-697, 2005
8. Hub+�-�lek Z: Emmonsiosis of wild rodents and insectivores in Czechland. J Wild Dis 35:243-249, 1999
9. Kwon-Chung KJ, Bennett JE: Infections due to miscellaneous molds. In: Medical Mycology, pp. 733-739. Lea & Febiger, Philadelphia, PA, 1992
10. Sigler L: Ajellomyces crescens sp. nov., taxonomy of Emmonsia spp., and relatedness with Blastomyces dermatitidis (telomorph Ajellomyces dermatitidis). J Med Vet Mycol 34:303-314, 1996
11. Sun Y, Bhuiya T, Wasil T, Macias A, Wasserman PG: Fine needle aspiration of pulmonary adiaspiromycosis. Acta Cytol 51:217-221, 2007