Signalment:
Two-month-old male African green monkey (
Chlorocebus
aethiops sabaeus).This animal was found dead in the enclosure on the
morning of the day of necropsy.
Gross Description:
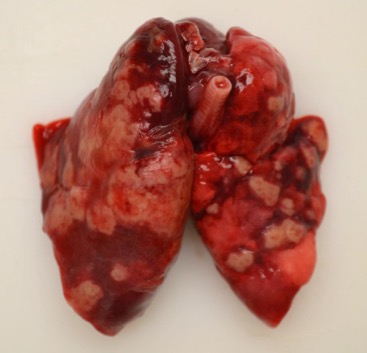
Approximately 75% of the left and 50% of the
right lung lobes were replaced by randomly scattered, multifocal to coalescing,
umbilicated, 3x3x3 mm to 1.5x3x2cm, firm, off-white to tan nodules, surrounded
by 1-5 mm wide dark red zones. The remainder of the left lung was dark red,
heavy and wet, and the lateral aspect was adhered to the adjacent costal
pleura. Neither side collapsed upon opening the thoracic cavity, and the left
lung sank in formalin.
Histopathologic Description:
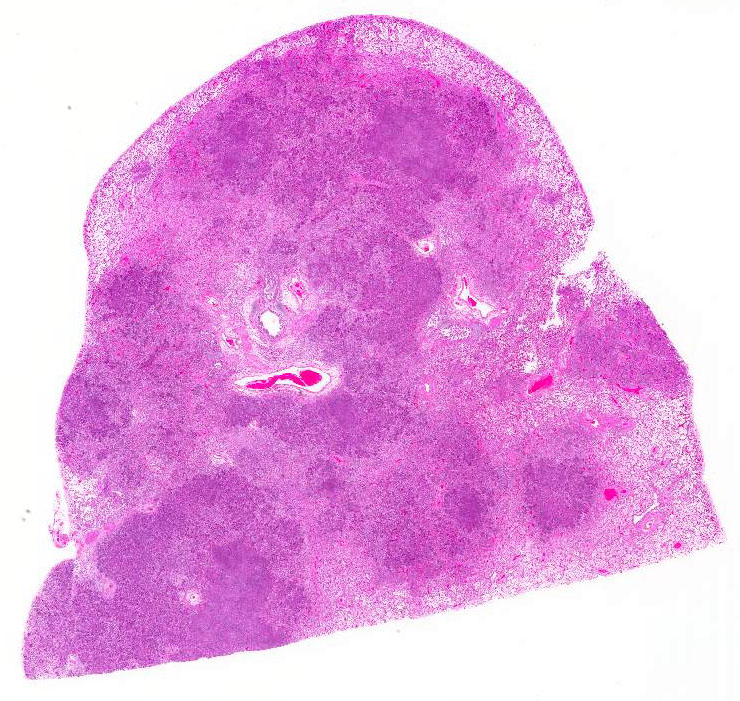
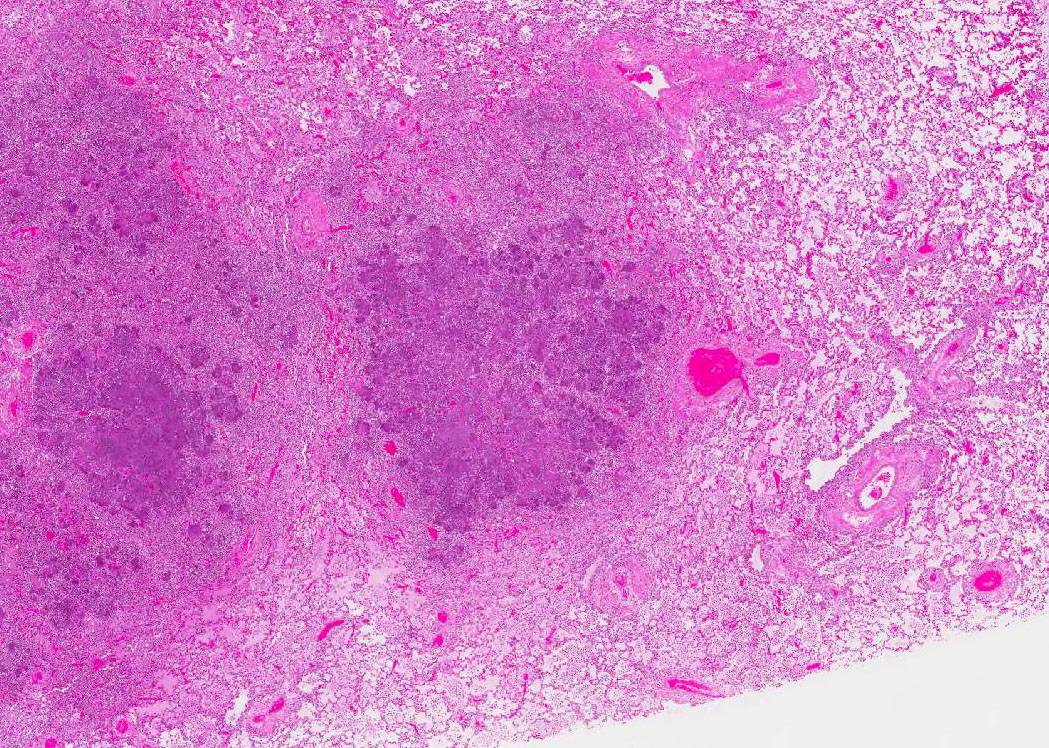
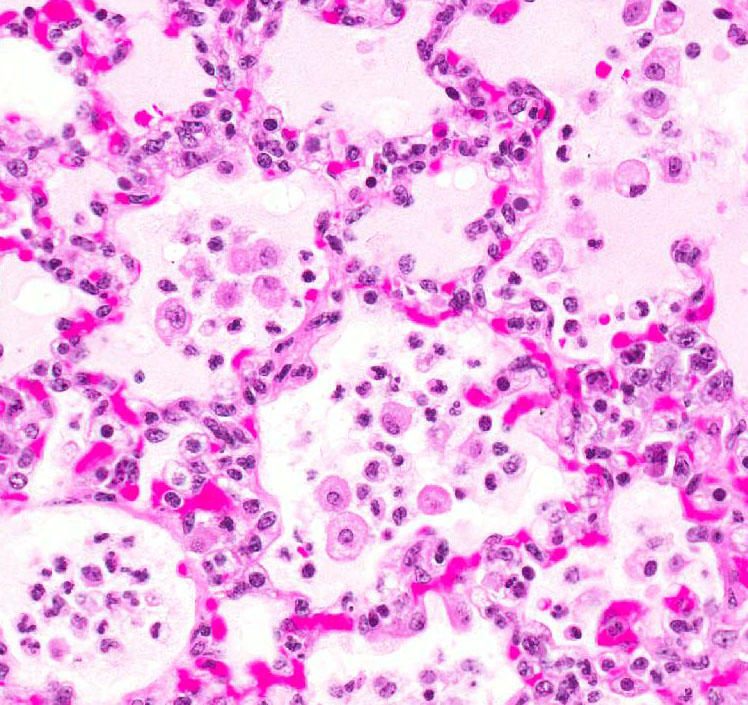
Most of the architecture is
effaced and replaced by fragmented eosinophilic cellular and karyorrhectic to
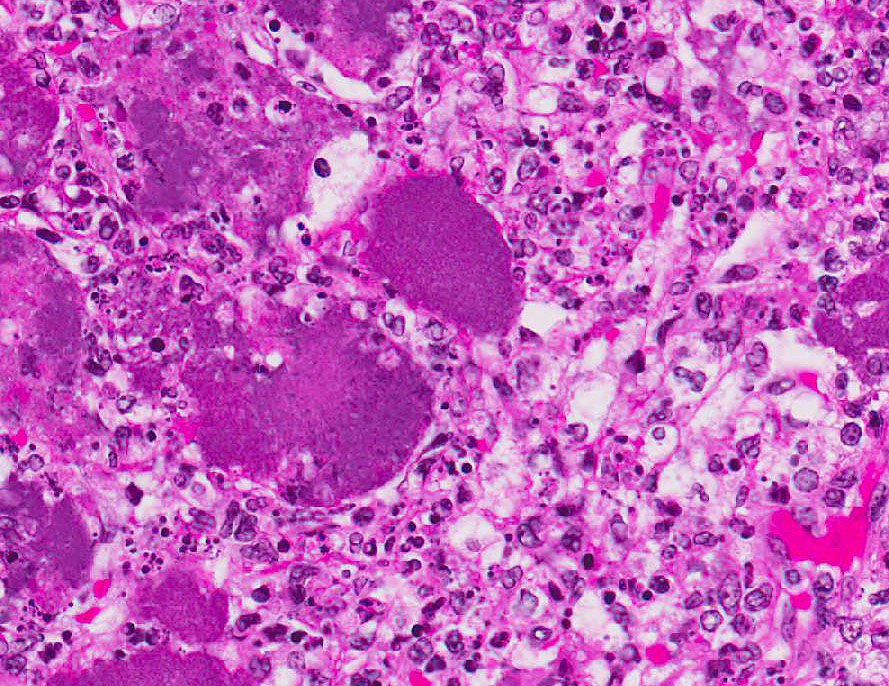
streaming nuclear debris (necrosis), innumerable neutrophils, many degenerate,
and large foamy macrophages. Colonies of small bacterial rods are prominent in
areas of necrosis. The wall of the left bronchus is transmurally effaced by
similar necrotic inflammatory exudate which extends into the lumen and also
lies within adjacent bronchioles. The remaining alveolar spaces are filled with
homogeneous proteinaceous material (edema fluid),
frequent neutrophils and foamy alveolar macrophages. Connective tissue fibers
surrounding the pulmonary vasculature are separated by clear space (edema).
Morphologic Diagnosis:
Pneumonia, multifocal,
chronic, severe, necrotizing with bacilli (consistent with
Yersinia
enterocolitica).
Lab Results:
Aerobic bacterial culture positive for
Yersinia enterocolitica.
Condition:
Necrotizing pneumonia/Yersinia enterocolitica
Contributor Comment:
Yersinia enterocolitica is a short gram-negative bacillus within the family
Entero-bacteriaceae.
It is an important food-borne pathogen that is commonly found in the
gastrointestinal tract of humans and other species. Pigs and rodents are
sources of human infection, and transmission occurs through contaminated food
and water. This pathogen most often produces necrotizing gastrointestinal
lesions with large colonies of bacteria in humans and animals, but can also
affect the urinary, respiratory, musculo-skeletal, integumentary and
cardiovascular systems in humans.
1,7.
Yersinia enterocolitica is
classified into 6 biotypes and 57O group strains. Among the 6 biotypes, all
except the 1A biotype (1B and 2-5) are pathogenic. 1B/O:8, 2/O:5, 2/O:9, 3/O:3
and 4/O:3 are most commonly isolated from humans. 1B/O:8 is considered the new
world strain and is highly pathogenic to humans and predominantly present in
the United States, while 4/O:3 and 2/O:9 are found in the Europe and Asia.
Strain 4/O:3 has frequently been isolated from pigs and 1B/O:8 has been
isolated from rodents.
1,3,7
Pathogenic strains of
Y. enterocolitica contain the
virulence plasmid pYV/pCD which encodes
Yersinia adhesin A (yadA),
Ysc-Yop type III secretion system (TTSS), chromosomally encoded virulence genes
ail, myfA, ystA, ysa, and the high pathogenicity island (HPI-) that aids in
iron acquisition. The expression of virulence genes depends on temperature and
calcium availability
in-vivo. Virulence proteins produced by PYV plasmid
resist phagocytic killing and complement mediated lysis of the bacteria by the
host.
Successful infection begins with
colonization of the distal small intestinal mucosa. Attachment and entry into
the intestinal epithelial cells are facilitated by yadA and
INV, respectively.
Upon entering the epithelial cells the bacteria preferentially enter M cells in
the Peyers patches where macrophages internalize them and transport them to
the mesenteric lymph nodes (MLN).
Yersinia bacteria multiply within the
macrophages and also extracellularly in the lymphoid organs leading to abscess
formation, often bacteremia and frequently, hepatitis.
1,3,7
This case
was the first in an outbreak involving more than 20
Y. enterocolitica
fatalities in 2015 2016, and was unique in that the pneumonia occurred in the
absence of intestinal and MLN lesions. The finding of necrotizing tracheitis
and large airway orientation in the lung suggests a respiratory route of
infection. Some reports in humans have also suggested primary infection by inhalation,
without gastrointestinal involvement.
2,9
JPC Diagnosis:
Lung: Pneumonia, necrosuppurative,
multifocal to coalescing, subacute, severe, with fibrin thrombi, hemorrhage,
edema, and numerous colonies of coccobacilli, African green monkey,
Chlorocebus aethiops sabaeus.
Conference Comment:
The
contributor provides a rare case of pneumonia caused by
Yersinia
enterocolitica in an African green monkey, as well as an excellent
discussion of the virulence factors and pathogenesis of the bacterium. Despite
the highly usual presentation, this case illustrates the characteristic
appearance of the gram-negative coccobacilli forming multifocal to coalescing
bacterial microcolonies surrounded by intense infiltrates of viable and
degenerate neutrophils and necrotic cellular debris filling and replacing
pulmonary architecture.
Yersinia sp. Micro-colonies are readily
distinguishable from other large colony-forming bacteria such as
Actinomyces, Actinobacillus, Coryne- bacterium, Staphylococcus and
Streptococcus spp.
Other species of Yersinia (pseudotuberculosis and
pestis) have the same histologic appearance in tissue section as is present in this
case. Speciation of the bacteria requires culture or bacterial isolation.
1,9
Yersinia
enterocolitica has a
worldwide distribution and affects a many different animal species and humans.
1,2,7,9
As mentioned by the contributor, the most common mode of transmission is caused
by foodborne outbreaks in humans. In nonhuman primates, infections are
typically acquired by the fecal-oral route.
8,9 The organism can be
shed in the feces by asymptomatic animals in the collection, as well as by
rodents and birds in the environment where it can survive and grow at low
temperatures. Disease is often secondary to stress, poor nutrition,
environmental flooding, and cold weather.
9 The contributor suggests
that the route of infection for this animal is via inhalation of the organism,
perhaps via aerosolized feces, causing the development of necrotizing
tracheitis and pneumonia without gastrointes-tinal lesions.
Many conference participants noted the presence of numerous large fibrin
thrombi within peribronchial veins and lymphatic vessels. Gram-negative
bacteria, such as
Yersinia spp, commonly cause sepsis and its associated
coagulopathy. During gram-negative septic shock, lipo-polysaccharide
(LPS, endotoxin) induces increased tissue factor (TF)
and factor XII expression.
4,5,6 TF (factor III) then forms a complex
with plasma factor VII as part of the extrinsic coagulation pathway. This complex activates factor X of the common pathway and factor
IX of the intrinsic pathway, leading to the formation of thrombin (factor II)
which generates fibrin from fibrinogen (factor I). Fibrin is cross-linked and
polymerized via factor XIII and deposited as a fibrin clot.
During sepsis, procoagulant activity predominates over
anticoagulation and fibrinolysis due to inhibition of tissue factor pathway
inhibitor (TFPI), thrombomodulin, and protein C as well as activation of
plasminogen activator inhibitor (PAI-1), and previously mentioned TF.5,6
This leads to disseminated intravascular coagulation (DIC) and exuberant fibrin
deposition. DIC eventually
consumes critical blood-clotting factors leading to hemorrhage and shock.5,6
However, a recent study in mice has shown that fibrin can also perform critical
protective functions during infection with Yersinia enterocolitica.
These studies suggest that fibrin may be an attempt by the host to physically
trap bacteria limiting dissemination, in addition to facilitating the
recruitment and activation of neutrophils and macrophages within infected
tissues via interleukin 6 (IL-6) and monocyte chemotactic protein-1 (MCP-1).5
In fact, mice treated with the anticoagulant warfarin prior to inoculation with
Yersinia enterocolitica had a markedly higher systemic bacterial burden
and shortened survival time compared to untreated mice.5
References:
1. Galindo
CL, Rosenzweig JA, Kirtley ML, Chopra AK. Pathogenesis of Y. enterocolitica
and Y. pseudo-tuberculosis in human yersiniosis. J Pathog 2011;
2011:182051.
2. Hosaka
S, Uchiyama M, Ishikawa M, Akahoshi T, Kondo H, Shimauchi C, et al.
Yersinia
entero-colitica serotype O:8 septicemia in an otherwise healthy adult:
analysis of chromosome DNA pattern by pulsed-field gel electrophoresis.
J Clin
Microbiol 1997; 35(12):3346-3347.
3. Kay
BA, Wachsmuth K, Gemski P, Feeley JC, Quan TJ, Brenner DJ. Virulence and
phenotypic characterization of
Yersinia enterocolitica isolated from
humans in the United States.
J Clin Microbiol 1983; 17(1):128-138.
4. Kumar
V, Abbas AK, Fausto N. Hemodynamic Disorders, Thromboembolic Disease, and
Shock. In:
Robbins and Cotran Pathologic Basis of Disease. 9th ed.
Philadelphia, PA:Elsevier Saunders; 2015:117-134.
5. Luo
D, Szaba FM, Kummer LW, et al. Protective roles for fibrin, tissue factor,
plasminogen activator inhibitor-1, and thrombin activatable
fibrinolysis inhibitor, but not factor XI, during defense against the
gram-negative bacterium Yersinia enterocolitica. J Immunol.
2011; 187(4):1866-1876.
6. Mosier
DA. Vascular disorders and thrombosis. In: McGavin, MD, ed.
Pathologic Basis
of Veterinary Disease. 6th ed. St. Louis, MO:Elsevier; 2017:51-72.
7. Sabina
Y, Rahman A, Ray RC, Montet D:
Yersinia enterocolitica: Mode of
transmission, molecular insights of virulence, and pathogenesis of infection.
J
Pathol. 2011; 2011:429069.
8. Simmons
J, Gibson S. Bacterial and mycotic disease of nonhuman primates. In: Abee CR,
Mansfield K, Tardif S, Morris T eds
. Nonhuman Primates in Biomedical
Research: Diseases. Vol 2. 2
nd ed. Philadelphia, PA: Elsevier;
2012:138-140.
9. Wong
KK, Fistek M, Watkins RR. Community-acquired pneumonia caused by
Yersinia
enterocolitica in an immunocompetent patient.
J Med Microbiol 2013;
62(4):650-651.
10. Uzal FA,
Plattner BL, Hostetter JM. Alimentary system In: Maxie MG, ed.
Jubb,
Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed.
Philadelphia, PA: Elsevier; 2016:176-177.