CASE II: 19-0830-64 (JPC 4161168)
Signalment:
Adult (3-year old) limousine female sheep (Ovis aries)
History:
A ewe from a flock of 300 meat sheep and 70 dairy sheep was presented for postmortem examination. Several animals (ewes and rams) were introduced into the flock during the previous years and months. The sheep were had been raised exclusively outdoors, in harvested fields and woods.
Over the past few months, 6 ewes developed similar symptoms, with chronic and progressive dyspnea, nasal discharge, characteristic stertorous breathing and increased appetite for salt. Progressive cachexia led to euthanasia. Three of these animals were necropsied and similar lesions were found.
Gross Pathology:
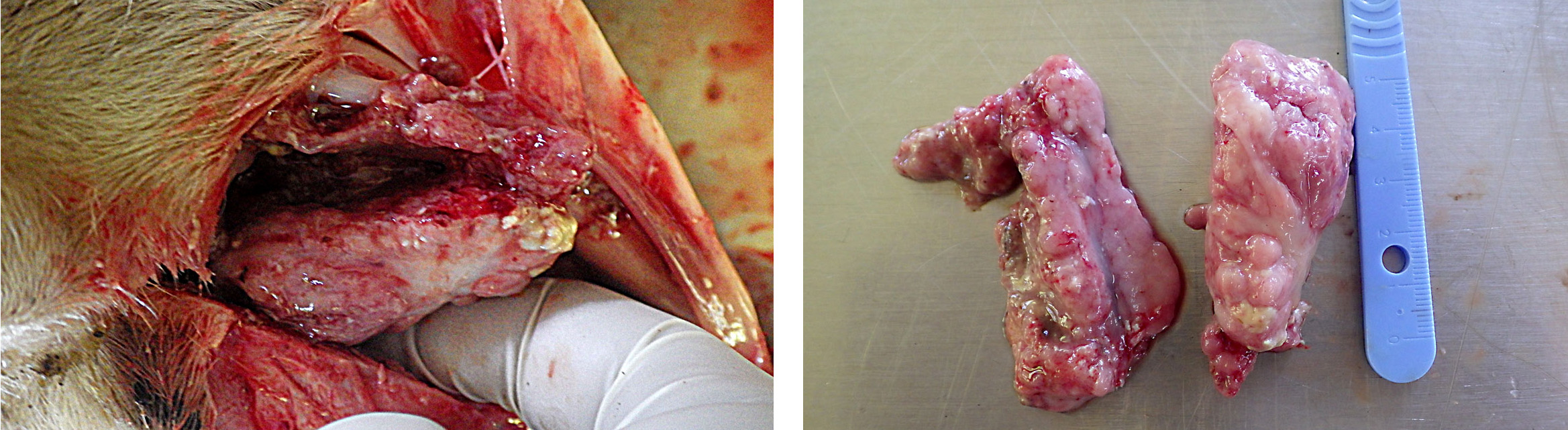
The animal was cachectic. The main gross lesion was a bilateral mass in the ethmoid region and slight protrusion into the nasopharynx. The mass was exophytic, about 6 cm wide, rather firm, with a lumpy, wet surface focally covered with fibrin. The diaphragm was thickened (hypertrophy), secondary to obstruction of the respiratory tract.
Laboratory results: None.
Microscopic Description:
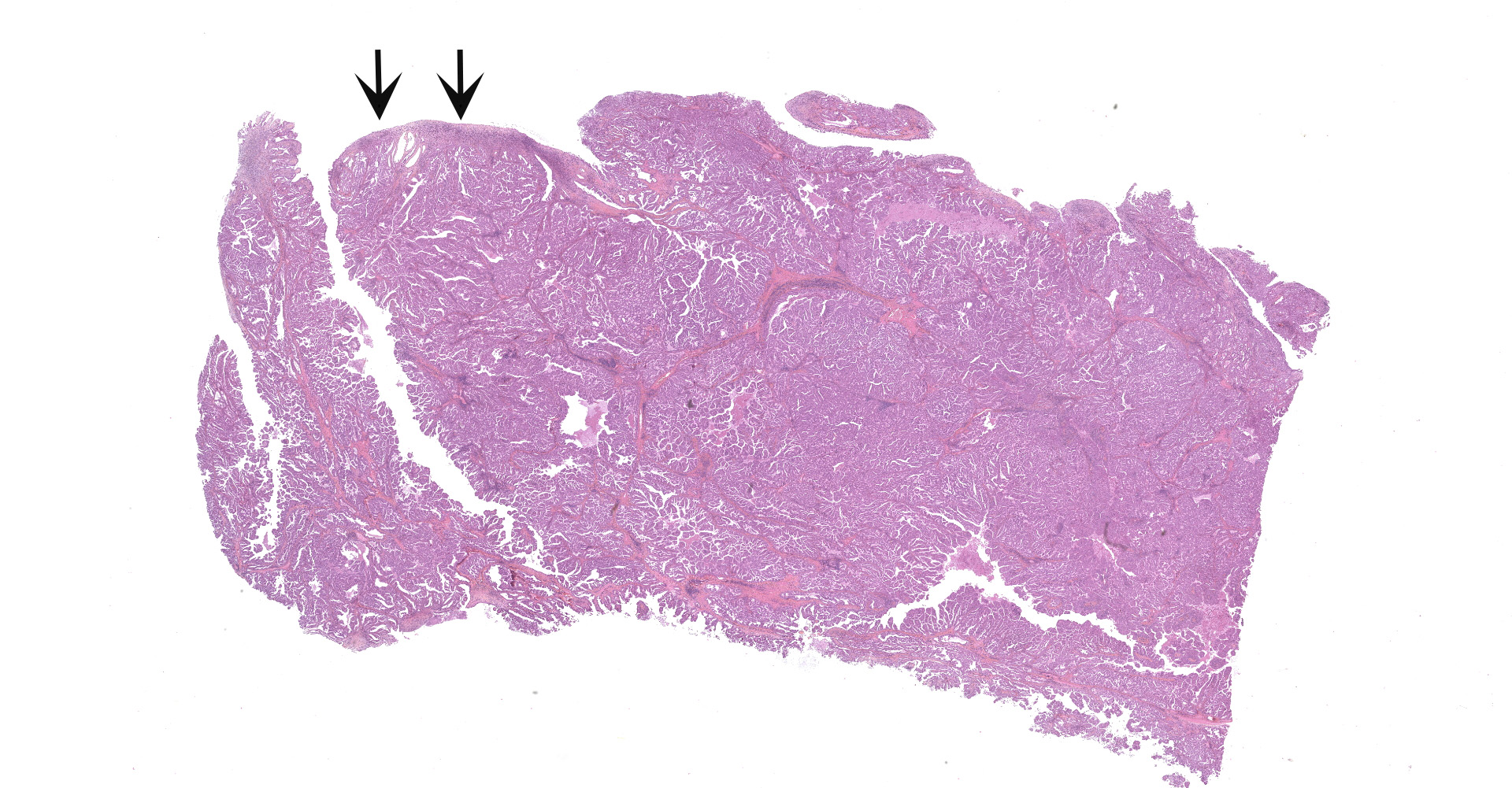
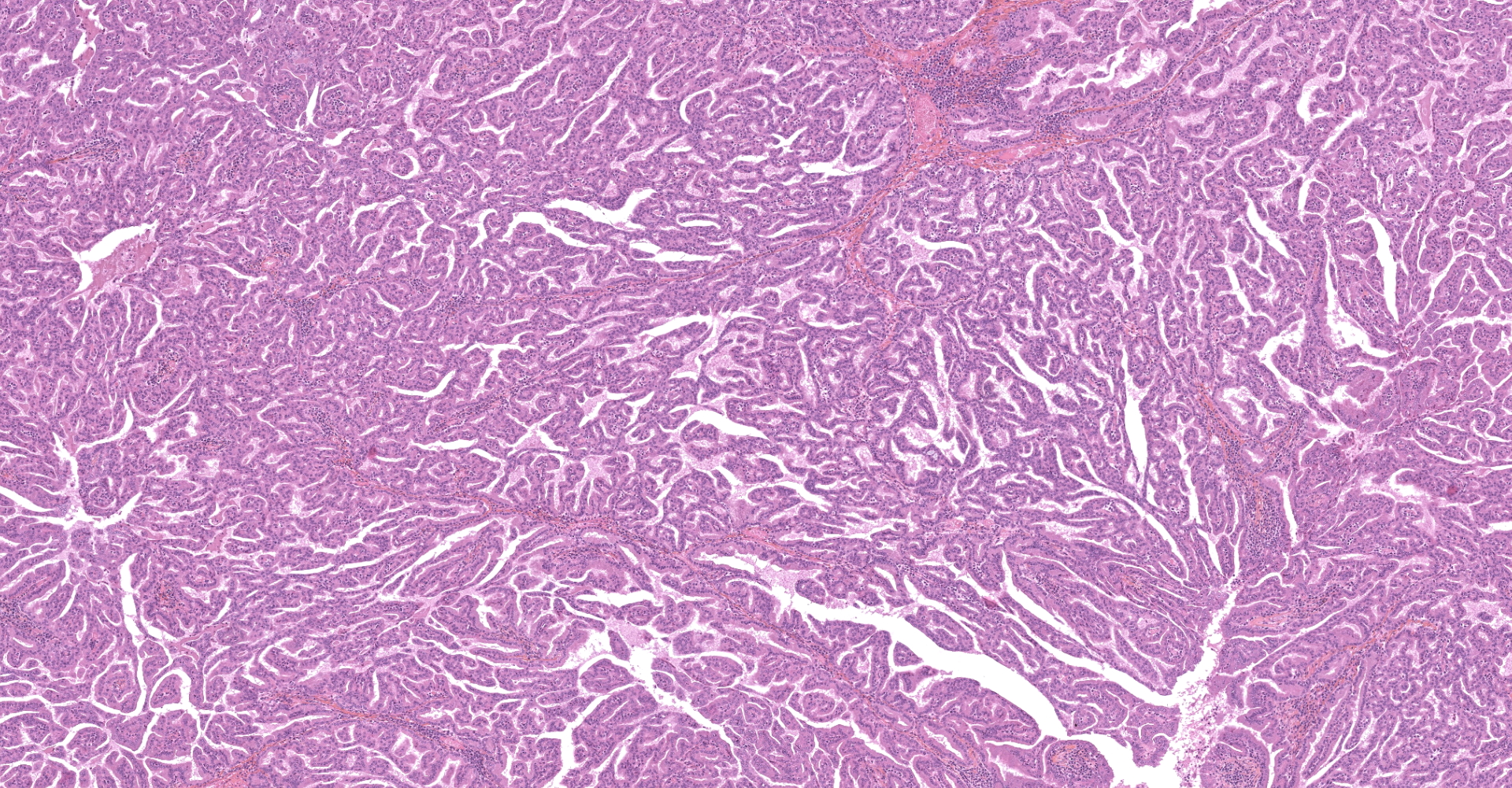
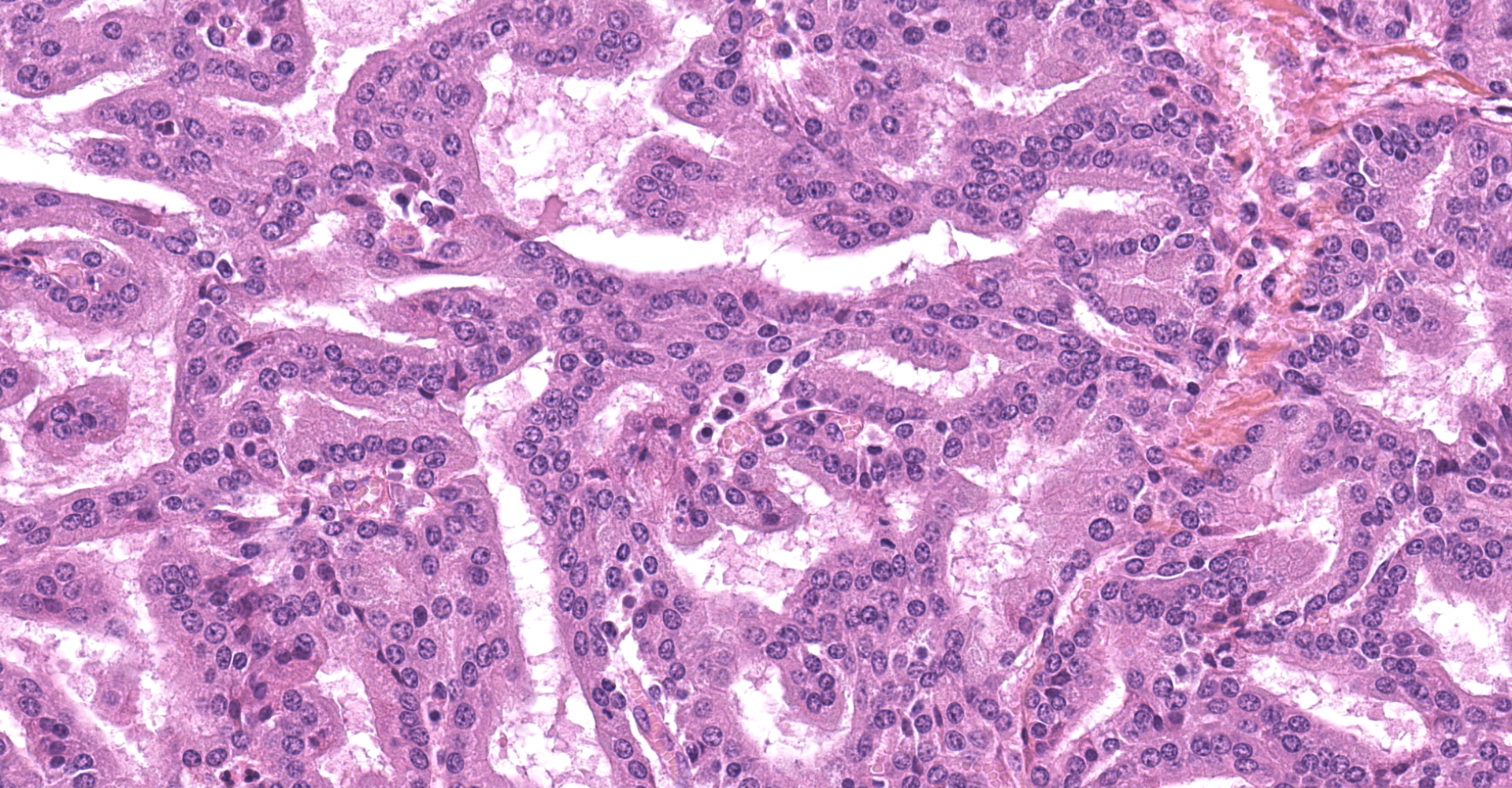
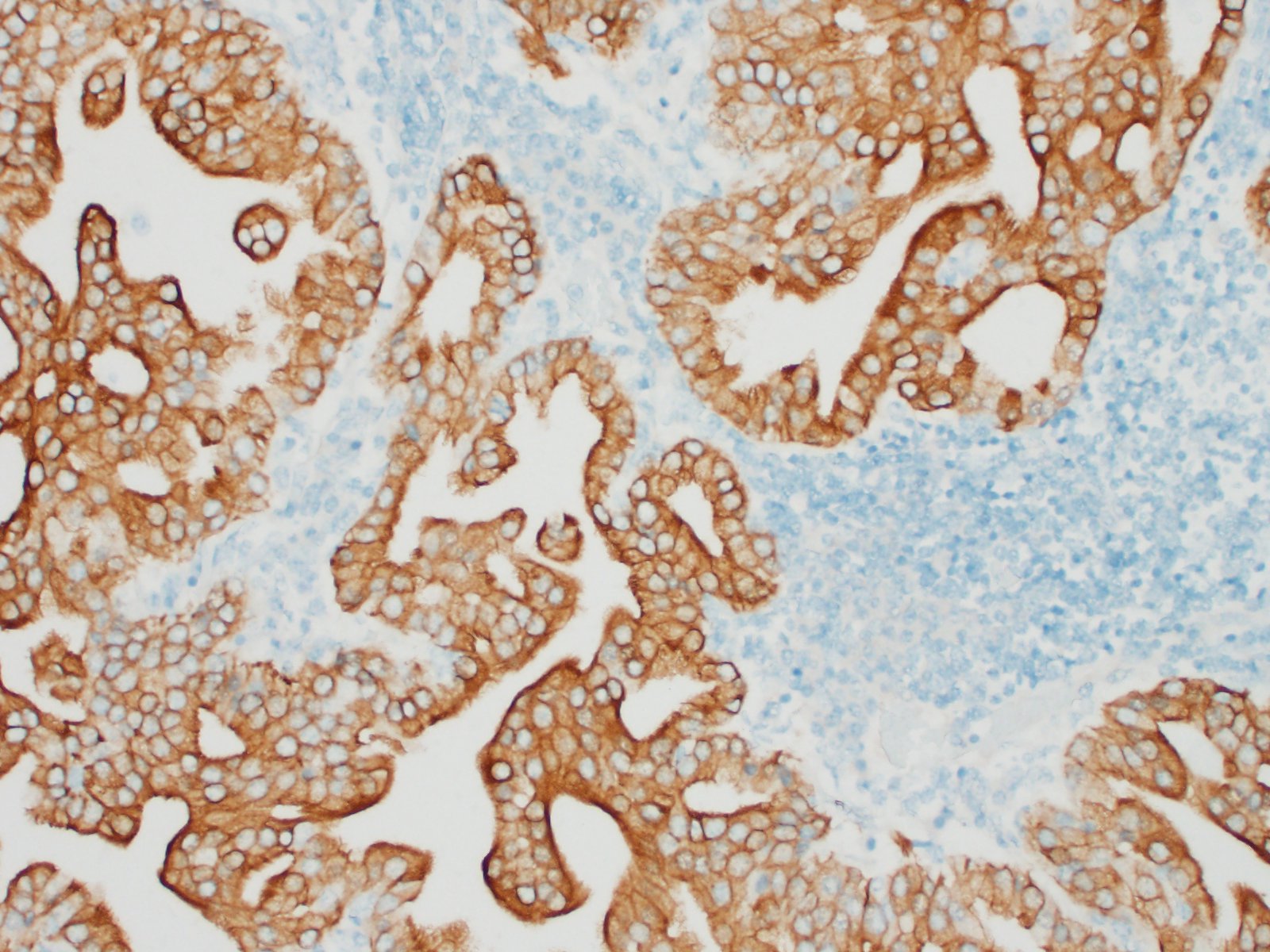
The submitted sample is neoplastic tissue with no normal structures. The tissue is grossly lobulated, composed of a tubulopapillar proliferation of rather monomorphous epithelial tumor cells. Tumor cells are medium-sized (25 µm wide), cuboidal when forming tubules and more prismatic or pseudostratified in papillary structures. Intercellular junctions are inapparent. Cytoplasm is abundant and eosinophilic, with small PAS-positive and Giemsa stain-metachromatic apical secretory granules (mucins). Nuclei are round, large, generally in a basal position, with a small nucleolus. Anisokaryosis is moderate. Mitotic figures are rare. Some mucin admixed with cell debris is present within glandular lumens. The stroma is scarce, focally infiltrated by lymphocytes and plasma cells. Small necrotic foci are present (more obvious in one of the submitted sections). No emboli could be observed.
Contributor 's Morphologic Diagnoses:
Enzootic nasal tumor.
Contributor 's Comment:
Although normal nasal mucosa is not present on slides, the presence of a tubulopapillary adenocarcinoma with mucin secretion in a sheep is suggestive of enzootic nasal tumors (ENTs). ENTs are transmissible epithelial tumors of sheep and goats, geographically widespread, except for Australia, New Zealand and United Kingdom8, with an incidence in affected flocks of 0.1 to 15%.1, 8 Adults and lambs older than 6 months can be affected.
The tumors arise from the ethmoid turbinates, fill the caudal nasal cavity and can be unilateral to bilateral.1 Their growth is compressive, leading to deviation of the nasal septum and facial deformity.8 The duration
of disease varies from 3 weeks to more than 1 year.7 In goats, edematous inflammatory polyps can grow proximally to the tumor and protrude from the nostrils.8 Those polyps can be more obvious than the tumor itself at necropsy and should not be misinterpreted.
Histologically, the tumor is a well-differentiated glandular tissue, with tubular, papillary and acinar arrangements made of cuboidal to pseudostratified, non-ciliated, epithelial cells. The fibro-vascular stroma is scant and variably infiltrated by lymphocytes. The tumor is histologically similar in sheep and goats, and considered as adenoma or as low-grade adenocarcinoma.8 Metastases are not reported.8
ENTs are caused by species specific retroviruses: ovine ENTV-1 and caprine ENTV-2. Both are closely related to the Jaagsiekte sheep retrovirus (agent of the ovine pulmonary adenocarcinoma) and to sheep endogenous retroviruses.2, 6 These are examples of retrovirus-induced epithelial tumors. Recently, a case of nasal adenocarcinoma associated with Jaagsiekte sheep retrovirus has been described in a sheep.4
Contributing Institution:
Ecole Nationale Vétérinaire d 'Alfort
Unité d 'Histologie et d 'Anatomie Pathologique, BioPôle Alfort Département des Sciences Biologiques et Pharmaceutiques 7 avenue du Général De Gaulle
94704 Maisons Alfort Cedex FRANCE
JPC Diagnosis:
Nasal cavity: Respiratory adenocarcinoma.
JPC Comment:
Peyton Rous successfully transmitted a tumor from a hen to a chicken in 1909 via injection of cell-free extracts, one of the first indications of the association between viruses and neoplasia. This discovery served as a starting point for additional discoveries over the following decades in the field of virology.5
ENTV-1, ENTV-2, and Jaagsiekte sheep retrovirus (JSRV), are oncogenic beta retroviruses. These enveloped RNA viruses depend on DNA-polymerase for their replication. In order to achieve this, the viral DNA or provirus integrates into the cellular genome during the early steps of the viral cycle and remain in the host DNA. Other oncogenic retroviruses include bovine leukemia virus, feline leukemia virus, avian leucosis virus (chickens), Rous sarcoma virus
(chickens), walleye dermal sarcoma virus, mouse mammary tumor virus, murine leukemia virus, koala retrovirus B, and human T-lymphotropic virus.5
The respiratory route of transmission of oncogenic retroviruses in small ruminants was reported as early as 1934. In addition, JRSV can infect animals very early in life, with virus detectable at birth, suggesting in utero transmission. JRSV has also been detected in colostrum, which suggests the virus can be spread to newborns via colostrum and milk. The incubation period in naturally infected animals range from months to two to four years. This may vary depending on the type of infection, experimental inoculation having a shorter incubation period in comparison to spontaneous infection.5
As noted by the contributor, a case of an ovine nasal adenocarcinoma associated with JSRV was recently identified in an 8 year old Belclare ewe in Ireland. The neoplasm had gross and microscopic features and immunohistochemistry results consistent with ENTV-1. However, differential PCR using primers specific to regions of divergent sequences between the viruses produced results negative for ENTV-1 and positive for JSRV. This was a significant finding, particularly given JSRV is endemic in sheep in the British Isles, whereas ENTV-1 has not been reported. Therefore, PCR in combination with immunohistochemistry is necessary to reach an accurate etiologic diagnosis, which is of significant importance in regions currently free of ENTV-1, such as the British Isles, Australia, and New Zealand.4
In addition to the aforementioned retroviral induced tumors, transmissible tumors affecting the paranasal sinuses of Rocky Mountain bighorn sheep have also been described. These tumors were previously hypothesized to be caused by ENTV-1, JSRV, or a closely related oncogenic retrovirus. However, screening via PCR and IHC of naturally occurring bighorn sheep sinus tumors were negative for these viruses and virus particles were not detected by electron microscopy in nasal secretions or cell cultures of sinus tumor tissues. Similar to ENTV-1, metastasis has not been reported with this entity. Transmissibility of the tumors was confirmed in 2016 after researchers inoculated tumor material and associated exudates from a naturally occurring sinus tumor into four Rocky Mountain Bighorn sheep lambs and four domestic lambs. Within 18 months of inoculation, all four inoculated domestic sheep (100%) and one of the four inoculated bighorn sheep (25%) developed tumors within the ethmoid sinuses or nasal conchae. The low transmission of sinus tumors to bighorns as compared to domestic sheep may suggest species differences in susceptibility to this disease. Therefore, measures to prevent transmission to domestic sheep may be warranted, including exclusion of domestic sheep from the natural range of bighorn sheep.3
There was spirited discussion amongst conference attendees in regard the classification of this neoplasm as a carcinoma rather than an adenocarcinoma due to the presence of neoplastic ciliated respiratory epithelial cells. Glandular epithelium is not typically ciliated and therefore "carcinoma" may be considered by some to be the more appropriate diagnosis.
References:
1. Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer 's Pathology of Domestic Animals. Vol 2. 6th ed. Elsevier; 2016:478- 480,560.
2. Cousens C, Minguijon E, Dalziel RG, et al. Complete Sequence of Enzootic Nasal Tumor Virus, a Retrovirus Associated with Transmissible Intranasal Tumors of Sheep. J Virol. 1999 May;73:3986?3993.
3. Fox KA, Wootton S, Marolf A, et al. Experimental Transmission of Bighorn Sheep Sinus Tumors to Bighorn Sheep (Ovis canadensis canadensis) and Domestic Sheep. Vet Pathol. 2016;53(6):1164-1171.
4. Jahns H, Cousens C. Nasal adenocarcinoma associated with jaagsiekte sheep retrovirus infection in a sheep. J VET Diagn Invest. 2019 Dec 30;32:152?155.
5. Monot M, Archer F, Gomes M, Mornex JF, Leroux C. Advances in the study of transmissible respiratory tumours in small ruminants. Vet Microbiol. 2015;181(1-2):170-177.
6. Ortín A, Cousens C, Minguijón E, et al. Characterization of enzootic nasal tumour virus of goats: complete sequence and tissue distribution. J Gen Virol. 2003 Aug;84:2245?2252.
7. Walsh SR, Stinson KJ, Menzies PI, Wootton SK. Development of an ante-mortem diagnostic test for enzootic nasal tumor virus and detection of neutralizing antibodies in host serum. 2014 Aug 1;95:1843?1854.
8. Wilson DW. Tumors of the respiratory tract. In: Donald J. Meuten, ed. Tumors in Domestic Animals. 5th ed. Wiley Blackwell; 2002:474?477.