CASE IV: 629-183 (JPC 4136414)
Signalment:
Adult, Musk lorikeet (Glossopsitta concinna)
History:
Wild bird found dead and submitted for necropsy examination.
Gross Pathology:
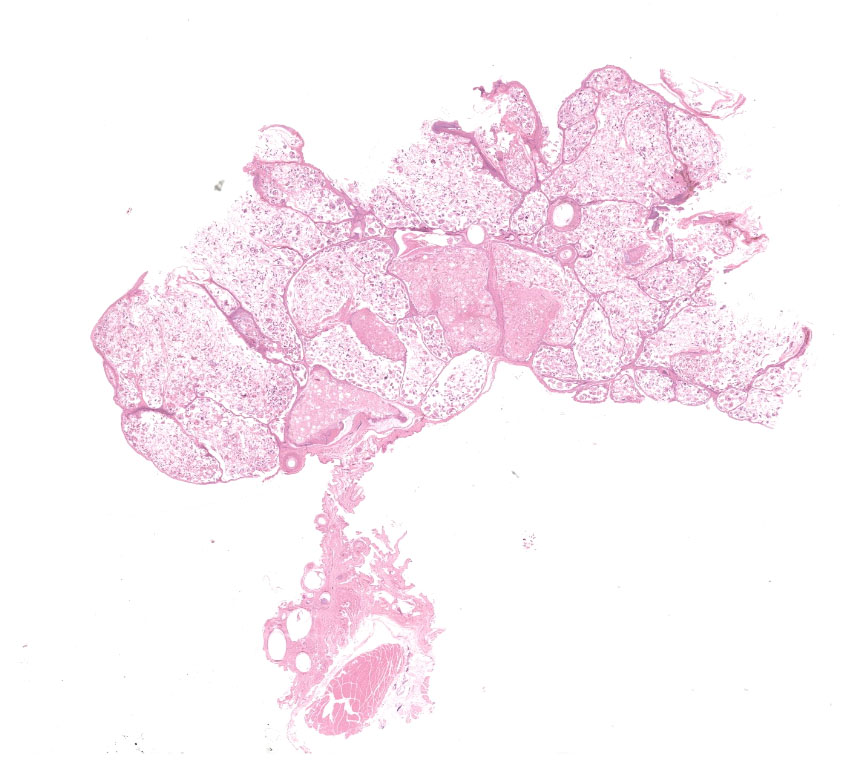
There were multiple white to beige dermal exophytic nodules with papillary surfaces arising from the skin in various locations on the body. The largest mass (40 x 30 x 20mm in size) was under the left wing, and smaller masses were present bilaterally on the right radius and ulna, and on the patagial skin. Further masses arose from the ventral neck (10 x 5 mm), the dorsal head (2 x 1mm), and adjacent to the cloaca (20 x 10 x 5 mm). The bird was in thin body condition, but no other findings were noted on gross examination.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
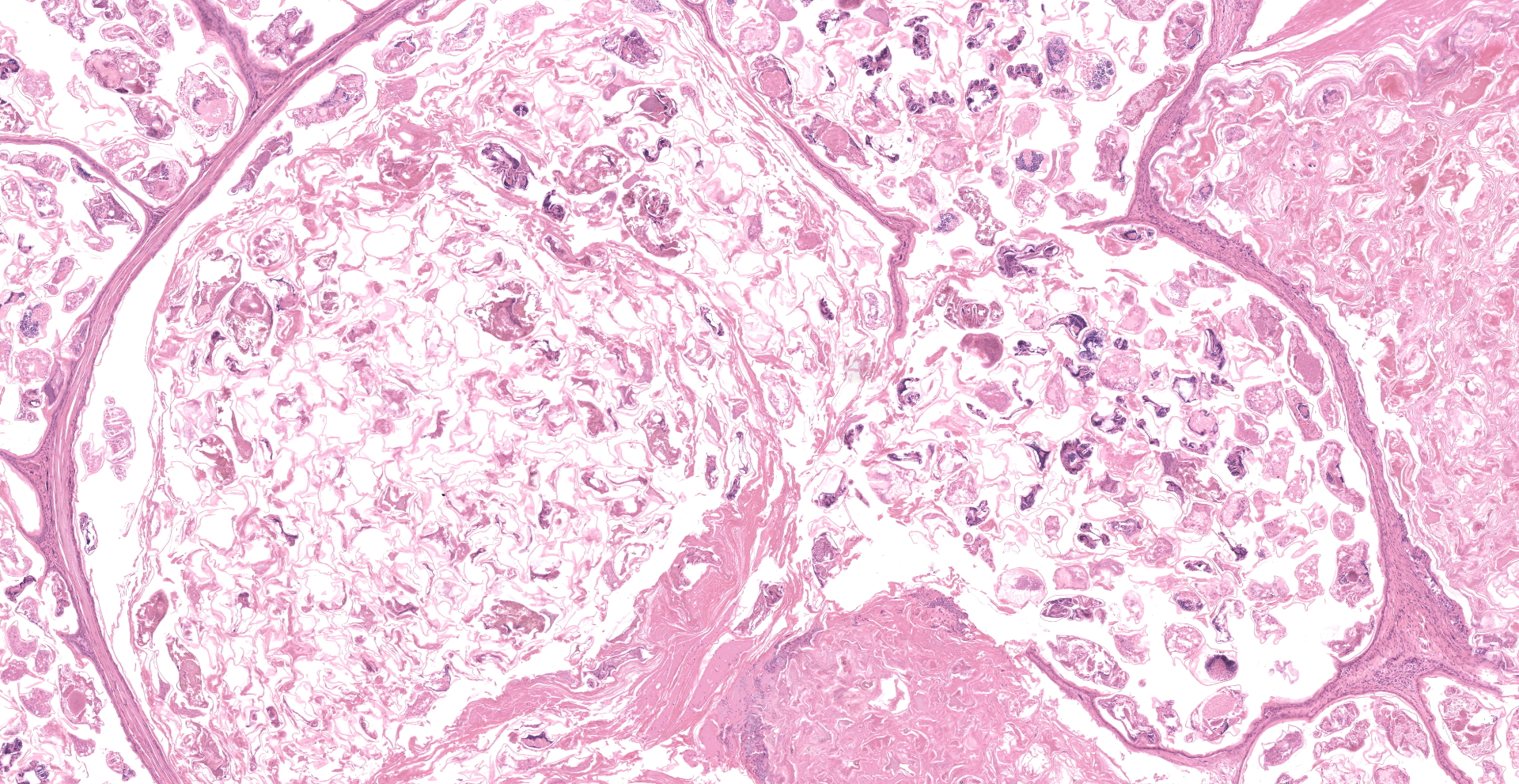
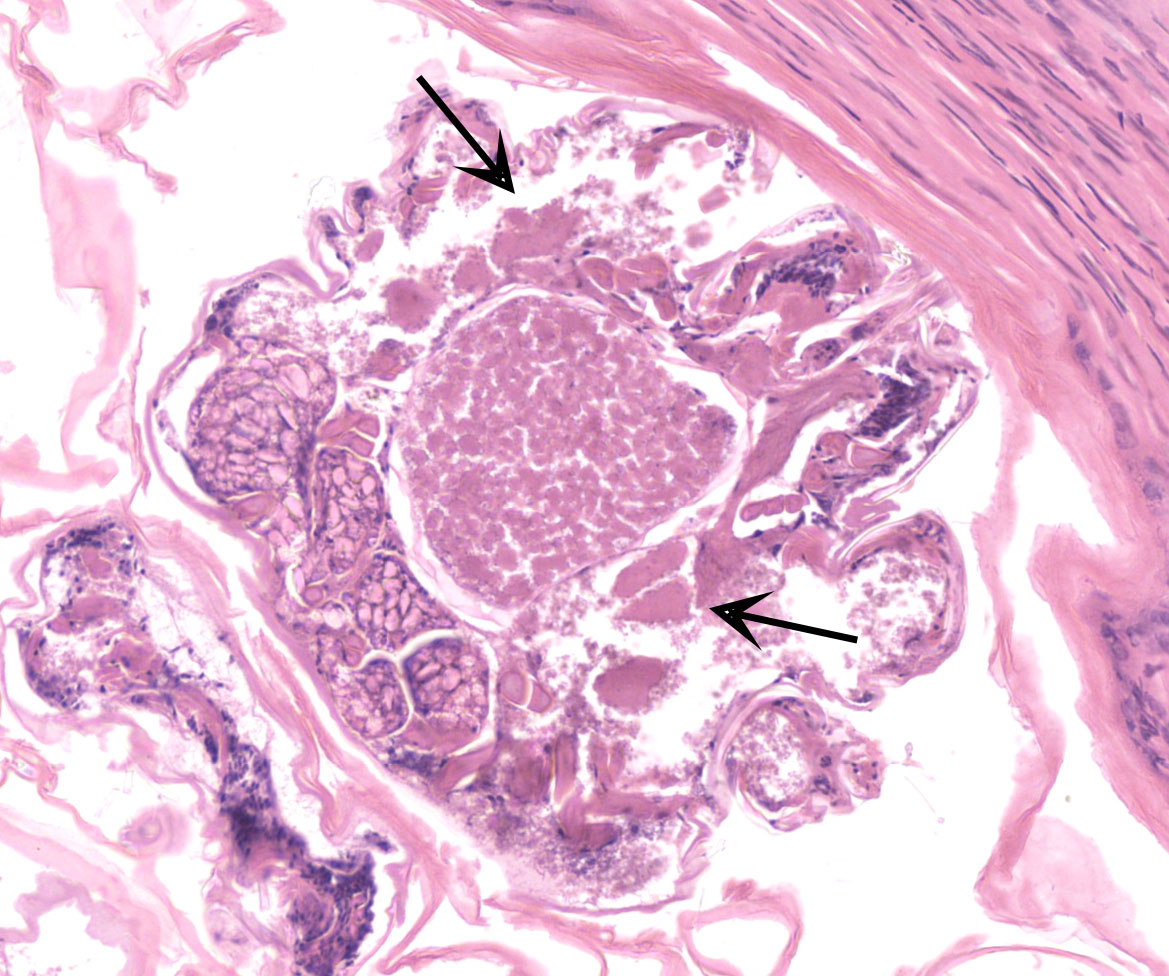
Feathered skin, dermis and subcutis: A multilocular cystic structure is expanding within the dermis and compressing adjacent structures, interspersed and supported by septa of fibrous connective tissue. The cysts are lined by thin stratified squamous epithelium, and the lumina of the structures are filled with abundant keratin. Embedded within the keratin, there are large number of arthropod cross sections characterized by a fragmented chitinous exoskeleton, partially intact, jointed appendages, a body cavity with striated muscle, multiple cross sections of gastrointestinal tract, and male and female reproductive tracts. Multifocally the lining of the cysts is eroded, and the surface is overlayed by a deposit of fibrin containing abundant degenerate heterophils. Occasional aggregates of cocci, bacilli, and yeasts are also present within some of the cystic structures.
Contributor's Morphologic Diagnoses:
Feathered skin, dermis and subcutis: Feather follicle cysts, multifocal, severe, chronic, with intralesional mites and mixed bacteria and yeasts.
Contributor's Comment:
The gross and histologic presentation in this case is compatible with feather follicle cysts caused by feather mites. Grossly, feather follicle cysts usually present as a bulge at the base of the feather, or as an oval or elongated dermal nodule or mass filled with yellow-whitish materials. They are most commonly found on the wings and over the back, but can appear anywhere on the integument. Such cysts can occur as a heritable condition of uncertain pathogenesis, especially in soft-feathered canaries, such as Gloucester and Norwich breeds,11 but the condition has also been reported in macaws, mynahs, and Amazon parrots.8 Feather cysts have also been found in parakeets and parrots as an acquired condition secondary to infection, trauma or any other condition that interferes with the feather growth.9
In this case, the formation of these cystic structure is caused by the accumulation of numerous arthropod mites within the feather follicle. The follicular epidermis of infested follicles proliferates to form a cystic structure which is distended with mites. Mites extracted from the follicle cysts in this case were identified as Harpyrhynchus rosellacinus according to the taxonomic keys of the prostigmatic mites (the location of Setae vi and ve; the characters of cuticle; and the segmentation of leg IV).2,7 The Harpyrhynchidae family is exclusive to birds,1 and species that have been discovered in Australia include H. kakatoe, H. monstrosus, and H. rosellacinus.2 Only H. rosellacinus is known to cause nodular lesions of the feather follicles in lorikeets, and it is the only species of mite known to cause feather cyst formation in birds.4,6 The mite has been found in the feather follicles of musk lorikeets (Glossopsitta concinna), eastern rosellas (Platycercus eximius), scaly-breasted lorikeets (Trichoglossus chlorolepidotus), and rainbow lorikeets (Trichoglossus moluccanus).2
Several species of feather mites are relatively common in birds, including blood sucking mites, such as Dermanyssus spp. and Ornithonyssus spp., which may cause anaemia and death in heavy infested cases;6 and non-pathogenic mites, such as Protolichus spp. and Dubininia spp., which feed on feather and skin debris only.5 Unfortunately, most types of mites cannot be by identified in histological section due to the lack of distinguishing morphologic characteristics.
Bacteria and yeasts (most consistent with Malassezia sp.) presents with mites in cysts are most likely an incidental finding in this case.
Contributing Institution:
JPC Diagnosis:
Feathered skin: Follicular ectasia, regionally extensive, marked, with innumerable mites, and ulcerative dermatitis.
JPC Comment:
Feather follicle cysts occur when a developing feather fails to breach the epidermis, resulting in the formation of a cyst as the feather continues to grow within the follicle. Therefore, any pathologic condition interfering with feather growth and/or emergence such as feather follicle dysplasia, endocrinopathy, neoplasia, malnutrition, superficial injury (e.g. feather picking), infestation, infection, or a combination of these factors predispose the bird to forming feather follicle cysts. Young birds are commonly affected by these benign lesions as their feathers initially emerge whereas older birds are more commonly affected as new feathers attempt to emerge during moulting, with the risk feather cyst development increasing with each subsequent moult.10
Also known as 'hypopteronosis cystica', 'pterylofolliculosis cystica', and plumafolliculomas, feather follicle cysts are relatively rare in domestic fowl species, while they're more common in small wild and caged birds. Feather follicle cysts exhibit a range of gross features and may be wet or dry, solitary or multiple, firm or soft, open or closed, fixated or sessile thin walled nodules measuring up to 4cm. When exposed, the luminal contents are typically composed of soft friable concentric lamellar structures centered on a malformed feather.10
Commonly known as 'quill mites', mites within order Prostigmata (including Harpyrhynchus spp.) and the superfamily Analgoidea (order Astigmata) infest multiple avian species, including gallinaceous birds, passerines, pigeons, ratites, parrots, and other psittacines. In some cases these mites are host specific whereas others infest multiple species. In contrast to other mites that feed on blood or sebaceous fluid, quill mites feed on the smooth, hollow, colourless part of the feather that does not have any barbs and inserts into the skin (i.e. the quill or calamus); they do not invade the rachis, a continuation of the quill to which the barbs attach, forming the vane. Clinical signs of quill mite infestation are typically mild and include irritation and pruritus, feather picking, and feather loss. With the aid of magnification, observation of mites within powdery quill material of malformed or broken feathers is typically diagnostic.3
During conference discussion, there was debate in how to best represent the primary process taking place in the tissue. A minority of participants favoured heterophilic folliculitis, whereas a majority favoured follicular ectasia as being the cause of the heterophilic folliculitis given the previously discussed pathogenesis associated with the formation of feather follicle cysts.
References:
1. Boyd EM. Two new species of harpyrhynchus from herons in north america (acarina, trombidiformes, harpyrhynchidae). Proc. Helm. Soc. Wash. 2012;35(1):18?24.
2. Domrow R. Acari Prostigmata (excluding Trombiculidae) parasitic on Australian vertebrates: an annotated checklist, keys and bibliography. Invertebr. Taxon. 1991;4:1283?1376.
3. Dorrestein GM, Van der Horst HH, Cremers HJ, Van der Hage M. Quill mite (Dermoglyphus passerinus) infestation of canaries (Serinus canaria): Diagnosis and treatment. Avian Pathol. 1997;26(1):195-199.
4. Filippich LJ, Domrow R. Harpyrhynchid mites in a scaly-breasted lorikeet, Trichoglossus chlorolepidotus (Kühl). J Wildl Dis. 1985;21(4):457-458.
5. Hinkle NC, Corrigan RM. External parasites and poultry pests. In: Swayne DE, ed. Diseases of poultry. 14th ed. Hoboken, NJ: Wiley Blackwell; 2020:1137-1156.
6. Ladds P. Arthropod and other ectoparasitic diseases in birds. In Ladds P, ed. Pathology of Australian Native Wildlife. Collingwood, VIC: CSIRO; 2009:385?390.
7. Perry RA, Gill J, Cross GM. Disorders of the avian integument. Vet Clin North Am Small Anim Pract. 1991;21(6):1307-1327.
8. Reavill DR, Schmidt RE. Avian surgical pathology. In: Fudge A, ed. Laboratory Medicine: Avian and Exotic Pets. Philadelphia, PA: Saunders; 2000:133?146.
9. Lawrence RF. A new mite parasite (harpyrhynchus) from the roselle parakeet (trombidiformes, acari). Proceedings of the Linnean Society of New South Wales. 1959;(84):238?241.
10. Samkange A, Mushonga B, Kandiwa E, Bishi AS, Segwagwe BVE, Muradzikwa E. A feather cyst causing vertebral bone lysis and spinal cord compression in a Lohmann Brown layer. J S Afr Vet Assoc. 2020;91(0):e1-e4.