Signalment:
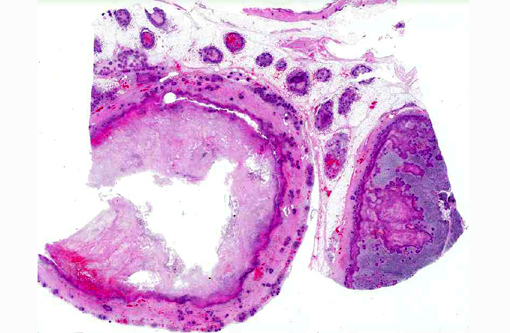
Gross Description:
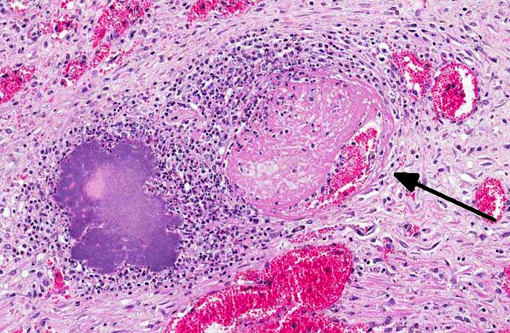
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
YPT, Yersinia enterocolitica (YE) and Yersinia pestis (YP) share 97% of their genomes as well as a tropism for lymph nodes. Despite this, manifestations of disease can be quite different for the three bacteria, presumably due to a combination of shared and unique chromosomal and plasmid-derived virulence factors.(3)
Less is known specifically about the pathogenesis of YPT infections than that of its close relative YE. As demonstrated with YE, following ingestion of contaminated food or water, bacteria adhere to distal small intestinal epithelial cells, cross the intestinal barrier using M cells which leads to bacterial replication in Peyers patches. Spread to mesenteric and other distant lymph nodes is common. There is little host response to bacterial replication in the first 36-48 hours following infection. Subsequently, an influx of activated phagocytes induces cytokine production and tissue necrosis. Septicemic spread from the distal ileum to the spleen and liver is relatively common.(3)
YPT is frequently isolated from feces of normal cattle. The organism may be shed in the feces by carrier animals in the group, or the environment may be contaminated by birds and rodents. The organism can survive and grow in cool environmental temperatures. Disease may be related to compromise of cell-mediated immunity and outbreaks may occur when animals are stressed (e.g., breeding, bad weather, poor nutritional condition). Clinical signs include mild diarrhea, severe hemorrhagic diarrhea, vague illness, or sudden death. Gross lesions may also be variable, with little noted in mild cases. More severe cases have hemorrhage in the intestine and enlarged hemorrhagic mesenteric lymph nodes. White foci of necrosis may be found in other organs, especially the liver and spleen.(1,2)
Diagnosis is based on the presence of characteristic lesions and isolation of the organism. Y. pseudotuberculosis prefers to grow at temperatures lower than the standard culture temperature of 37 degrees C, and since it may not be isolated using routine culture techniques, the laboratory should be notified when infection with this agent is suspected.(1,2)
Y. pseudotuberculosis also causes acute gastroenteritis and mesenteric lymphadenitis in humans. It is a food borne illness, and in the United States is much less common than Yersinia enterocolitica. In a review of FoodNet sites covering the period 1996-2007, 18 confirmed cases were identified, with an average annual incidence of 0.04 cases per 1,000,000 persons. Cases are probably underreported because less invasive forms are not recognized, and because of culture requirements for this organism. Infections in humans are less common in the United States than other countries.(4)
Our experience with this organism is similar to what is reported in the literature. We generally have seen sporadic cases, but this blackbuck was a case in an outbreak of 15 animals located in multiple enclosures, most of which are large and house multiple species. Attempts to isolate YPT from non-collection animals (e.g., wild rabbits and birds) were unsuccessful. In this blackbuck YPT was also isolated from the uterus, and mild fibrinous endometritis was present. Inflammation with bacteria was also seen in lung, liver, spleen, and urinary bladder.Â
JPC Diagnosis:
Conference Comment:
Next, the conference moderator expanded upon the contributors adept description of the pathogenesis of Yersinia pseduotuberculosis (YPT) and participants further analyzed several of its virulence factors. Transmission of YPT is generally fecal-oral, with rare transmission via inhalation. Yersinia adhesion A protein, or YadA (encoded on the pYV virulence plasmid) and invasin (encoded by the chromosomal inv locus) facilitate bacterial contact for invasion of host small intestinal M-cells; YadA adheres to extracellular matrix proteins such as fibronectin, collagen and laminin, while invasin binds to host cell b1 integrins. Next, Yops (Yersinia outer membrane proteins) come into play. YopB & YopD form a pore in the host cell membrane which allows the type three secretion system (T3SS) injectisome, which is also encoded on the pYV virulence plasmid and has a structure similar to flagella, to inject effector Yops into the host cell.(3)
The RNA chaperone protein Hfq is important in the production of T3SS effectors as well as intracellular survival. The PhoP/Q system (chromosomally encoded) stimulates growth and survival in macrophages as well as delayed macrophage activation via reduction in the stimulatory capacity of LPS. Superantigenic toxin YPM contributes to systemic infection by inducing T-cell proliferation and cytokine production. Various Yop proteins inhibit the host inflammatory response via alteration of phagocyte function, inhibition of signal transduction and disruption of the cytoskeleton. YopM sequesters caspase-1, which blocks activation of pro-inflammatory cytokines and YadA inhibits the classic complement and lectin pathways. YopP/J induces apoptosis of host cells by inhibiting the inflammatory and pro-survival actions of LPS and directly activating caspases. So, overall, YPT infections are characterized by an initial quiet 3648 hour period of bacterial replication with apoptosis and minimal host response, followed by a flood of phagocytic cells and an acute inflammatory response characterized by cytokine production and tissue necrosis.(3) This is illustrated by the abundant necrosis and large colonies of bacilli exhibited histologically.Â
To close, participants briefly discussed the role of exogenous superantigen in infection and immunity (such as the YPM toxin produced by Y. pseudotuberculosis in this case). Superantingens, which are often produced by Gram-negative bacteria, bind the Vb domain of the T-lymphocyte receptor (TCR) with the a chain of a class II major histocompatibility complex (MHC). This occurs outside of the normal antigen binding site and results in polyclonal T-lymphocyte activation regardless of antigen specificity, as well as massive cytokine release.(5)
References:
2. Valli VEO. Hematopoietic system. In: Maxie, MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed., vol 3. Philadelphia, PA: Elsevier; 2007:298-299.
3. Galindo CL, Rosenzweig JA, Kirtley ML, Chopra AK. Pathogenesis of Y. enterocolitica and Y. pseudotuberculosis in human yersiniosis. J Pathog. 2011:doi:10.4061/2011/182051.
4. Long C, Jones TF, et al. Yersinia pseudotuberculosis and Y. enterocolitica infections, FoodNet, 1996-2007. Emerg Infec Dis. 2010;16(3):566-567.
5. Snyder PW. Diseases of immunity. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2007:270.